Abstract
-
▴ Ciclesonide nasal spray delivers the corticosteroid ciclesonide as a hypotonic spray via a metered-dose manual pump.
-
▴ Systemic exposure to ciclesonide and its active metabolite desisobutyryl-ciclesonide is low after intranasal administration. High protein binding (≈99%) and rapid first-pass clearance further reduce systemic exposure to the drug.
-
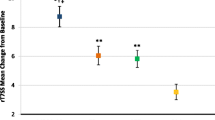
▴ In well designed trials, intranasal ciclesonide 200 μg once daily for 2–4 weeks was more effective than placebo in terms of improving nasal symptoms in adolescents and adults with moderate to severe seasonal allergic rhinitis. Quality of life measures were statistically significantly improved in ciclesonide relative to placebo recipients during the first 2 weeks of therapy.
-
▴ Similarly, in adolescents and adults with moderately severe perennial allergic rhinitis, ciclesonide 200 μg once daily was more effective than placebo in terms of reducing nasal symptoms in well designed trials of 6 weeks' and 1 year's duration. Improvements relative to placebo in quality of life measures were not considered clinically relevant.
-
▴ Ciclesonide nasal spray was generally well tolerated in these clinical trials; most adverse events were mild to moderate in intensity.



Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Nathan RA. The burden of allergic rhinitis. Allergy Asthma Proc 2007 Jan-Feb; 28(1): 3–9
Bousquet J, Khaltaev N, Cruz A, et al. Allergic Rhinitis and its Impact on Asthma: ARIA report 2007 [online]. Available from URL: http://www.whiar.org/docs/ARIA_WR_wm.pdf [Accessed 2008 Mar 12]
Price D, Bond C, Bouchard J, et al. International Primary Care Respiratory Group (IPCRG) Guidelines: management of allergic rhinitis. Prim Care Respir J 2006 Feb; 15(1): 58–70
Meltzer EO. Formulation considerations of intranasal cortico-steroids for the treatment of allergic rhinitis. Ann Allergy Asthma Immunol 2007 Jan; 98(1): 12–21
Nycomed US Inc. Omnaris (ciclesonide) nasal spray, 50 meg: US prescribing information [online]. Available from URL: http://www.fda.gov/cder/foi/label/2007/0221241bl.pdf [Accessed 2008 Mar 12]
FDA. Medical review: Omnaris nasal spray [online]. Available from URL: http://www.fda.gov/cder/foi/nda/2006/022004s000_MedR.pdf [Accessed 2008 Mar 13]
Sato H, Nave R, Nonaka T, et al. In vitro metabolism of ciclesonide in human nasal epithelial cells. Biopharm Drug Dispos 2007 Jan; 28(1): 43–50
Belvisi MG, Bundschuh DS, Stoeck M, et al. Preclinical profile of ciclesonide, a novel corticosteroid for the treatment of asthma. J Pharmacol Exp Ther 2005 Aug; 314(2): 568–74
Winkler J, Hochhaus G, Derendorf H. How the lung handles drugs: pharmacokinetics and pharmacodynamics of inhaled corticosteroids. Proc Am Thorac Soc 2004; 1(4): 356–63
Silvestri M, Serpero L, Petecchia L, et al. Cytokine-activated bronchial epithelial cell pro-inflammatory functions are effectively downregulated in vitro by ciclesonide. Pulm Pharmacol Ther 2006; 19(3): 210–7
Stoeck M, Riedel R, Hochhaus G, et al. In vitro and in vivo anti-inflammatory activity of the new glucocorticoid ciclesonide. J Pharmacol Exp Ther 2004 Apr; 309(1): 249–58
Ratner PH, Wingertzahn MA, van Bavel JH, et al. Efficacy and safety of ciclesonide nasal spray for the treatment of seasonal allergic rhinitis. J Allergy Clin Immunol 2006; 118(5): 1142–8
Meltzer EO, Kunjibettu S, Hall N, et al. Efficacy and safety of ciclesonide, 200 μg once daily, for the treatment of perennial allergic rhinitis. Ann Allergy Asthma Immunol 2007 Feb; 98(2): 175–81
Schmidt BM, Timmer W, Georgens AC, et al. The new topical steroid ciclesonide is effective in the treatment of allergic rhinitis. J Clin Pharmacol 1999 Oct; 39(10): 1062–9
Rohatagi S, Luo Y, Shen L, et al. Protein binding and its potential for eliciting minimal systemic side effects with a novel inhaled corticosteroid, ciclesonide. Am J Ther 2005; 12(3): 201–9
Derendorf H, Nave R, Drollmann A, et al. Relevance of pharmacokinetics and pharmacodynamics of inhaled corticosteroids to asthma. Eur Respir J 2006 Nov; 28(5): 1042–50
Hochhaus G, Wu K, Blomgren AL, et al. How do differences in tissue and protein binding affect pulmonary pharmacodynamics: ciclesonide vs budesonide [abstract no. A189]. 103rd International Conference of the American Thoracic Society; 2007 May 18–23; San Francisco (CA)
Nave R, Wingertzahn MA, Brookman S, et al. Safety, tolerability, and exposure of ciclesonide nasal spray in healthy and asymptomatic subjects with seasonal allergic rhinitis. J Clin Pharmacol 2006 Apr; 46(4): 461–7
Ratner P, Darken P, Wingertzahn M, et al. Ciclesonide and beclomethasone dipropionate coadministration: effect on cortisol in perennial allergic rhinitis. J Asthma 2007; 44(8): 629–33
Kim K, Quesada J, Szmaydy-Rikken N, et al. Intranasal ciclesonide coadministration with inhaled fluticasone propionate-salmeterol does not suppress cortisol in allergic rhinitis patients. J Asthma 2007 Sep; 44(7): 515–20
Nave R, Bethke TD, van Marie SP, et al. Pharmacokinetics of [14C]ciclesonide after oral and intravenous administration to healthy subjects. Clin Pharmacokinet 2004; 43(7): 479–86
Wingertzahn M A, Takanashi K, Nagano A. Persistence and effusion clearance to esophagus of ciclesonide in hypotonic and isotonic suspensions [abstract no. 1008]. J Allergy Clin Immunol 2006 Feb; 117 (2 Suppl. 1): S260. Plus poster presented at the 62nd Annual Meeting of the American Academy of Allergy, Asthma and Immunology; 2006 Mar 3–8; Miami Beach (FL)
Peet CF, Enos T, Nave R, et al. Identification of enzymes involved in phase I metabolism of ciclesonide by human liver microsomes. Eur J Drug Metab Pharmacokinet 2005; 30(4): 275–86
Rohatagi S, Arya V, Zech K, et al. Population pharmacokinetics and pharmacodynamics of ciclesonide. J Clin Pharmacol 2003 Apr; 43(4): 365–78
Nave R, Drollmann A, Steinijans VW, et al. Lack of pharmacokinetic drug-drug interaction between ciclesonide and erythro-mycin. Int J Clin Pharmacol Ther 2005 Jun; 43(6): 264–70
Ratner PH, Wingertzahn MA, van Bavel JH, et al. Effectiveness of ciclesonide nasal spray in the treatment of seasonal allergic rhinitis. Ann Allergy Asthma Immunol 2006 Nov; 97(5): 657–63
Chervinsky P, Kunjibettu S, Miller DL, et al. Long-term safety and efficacy of intranasal ciclesonide in adult and adolescent patients with perennial allergic rhinitis. Ann Allergy Asthma Immunol 2007 Jul; 99(1): 69–76
Acknowledgements and Disclosures
The manuscript was reviewed by: G. Hochhaus, Department of Pharmaceutics, University of Florida, Gainesville, Florida, USA; R.A. Nathan, Asthma and Allergy Associates, PC and Research Center, Colorado Springs, Colorado, USA.
The preparation of this review was not supported by any external funding. During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dhillon, S., Wagstaff, A.J. Ciclesonide Nasal Spray. Drugs 68, 875–883 (2008). https://doi.org/10.2165/00003495-200868060-00009
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200868060-00009