Summary
Abstract
Insulin lispro, alone (Humalog®) or as premixture (Humalog Mix25® or Humalog Mix50®) is indicated for the treatment of hyperglycaemia in diabetes mellitus in many countries worldwide. It is a recombinant human insulin analogue and, except for the transposition of two amino acids, is identical to endogenous human insulin. Insulin lispro has a faster onset of action and shorter duration of activity than regular human insulin, and the time-action profile of insulin lispro mimics that of the physiological response of endogenous human insulin to food intake. In diabetic patients, from young children to the elderly, it has demonstrated postprandial blood glucose control similar to or better than that achieved with regular human insulin, without an increased risk of hypoglycaemia. In some trials, the risk of hypoglycaemia, including nocturnal episodes, was less in insulin lispro recipients than in regular human insulin recipients. Insulin lispro alone, or as a premixture with the longer-acting insulin neutral protamine lispro, can be administered immediately before or after meals. This convenient and flexible injection schedule may enable patients, including those with a non-routine lifestyle or unpredictable eating or exercising habits, to achieve the tight glycaemic control required to minimise long-term complications of diabetes and contributes to patient satisfaction with treatment.
Pharmacologica Properties
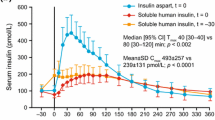
Insulin lispro is a recombinant human insulin analogue that is equipotent on a molar basis to regular human insulin with respect to hypoglycaemic activity, but with a faster onset of action, an earlier peak and a shorter duration of action. The blood glucose profile achieved with insulin lispro indicates that the optimal time of administration of insulin lispro is within 15 minutes before ingestion of a meal, but it can also be administered immediately or shortly after a meal. As a result of its shorter duration of action, insulin lispro is associated with lower postprandial, but higher postabsorptive and preprandial blood glucose levels, than regular human insulin, resulting in similar overall glycaemic control for the two insulins.
Commercially available premixtures of 25% insulin lispro and 75% insulin neutral protamine lispro (insulin lispro Mix25) and 50% of each (insulin lispro Mix50) have been shown to provide better 24-hour or mealtime glycaemic control than mixtures of human regular insulin with neutral protamine Hagedorn (NPH) insulin.
The incidence of specific or cross-reacting insulin antibodies in patients with type 1 or type 2 diabetes treated with insulin lispro was similar to that in patients treated with recombinant regular human insulin.
Therapeutic Efficacy
In patients with type 1 diabetes receiving insulin, intensive premeal administration of insulin lispro reduced mean 2-hour postprandial glucose levels relative to a similar regular human insulin-based regimen, but fasting or preprandial blood glucose levels were generally similar for the two insulins. Overall, long-term glycaemic control (as assessed by glycosylated haemoglobin [HbA1c] values) was essentially similar for the two insulins. The risk of hypoglycaemia was not greater with insulin lispro than with regular human insulin and was significantly lower in some trials.
Insulin lispro was also effective in the treatment of type 2 diabetes. In patients with early type 2 disease who had not previously used insulin, insulin lispro prompted a significantly greater reduction from baseline in mean overall 2-hour postprandial blood glucose excursions than glibenclamide (glyburide). The addition of insulin lispro to an oral antihyperglycaemic or NPH insulin regimen was effective at improving or regaining blood glucose control (reducing postprandial blood glucose and HbAic values) in patients with secondary oral antihyperglycaemic treatment failure. Patients with type 2 diabetes who were already receiving insulin therapy had 2-hour postprandial blood glucose levels or blood glucose excursions with insulin lispro that were generally significantly lower than with regular human insulin. Glycaemic control (HbAic values) and the incidence of hypoglycaemic episodes did not generally differ significantly between the insulin lispro and the other treatment groups in these trials.
Premixed insulin lispro achieved glycaemic control in patients with either type of diabetes that was similar to that with self-mixed insulin lispro and NPH insulin, or premixed or self-mixed regular human insulin and NPH insulin, with a generally similar risk of hypoglycaemia. In patients with type 2 diabetes, insulin lispro Mix25 twice daily and insulin lispro Mix50 three times daily demonstrated greater efficacy (significantly lower HbA1c values and postprandial blood glucose levels) than insulin glargine (a long-acting insulin) at bedtime. Twice-daily insulin lispro Mix25 demonstrated similar efficacy to twice-daily biphasic insulin aspart. In patients (including the elderly) with type 2 diabetes who had been previously inadequately controlled on a sulfonylurea, glycaemic control was significantly better with insulin lispro Mix25 than with oral glibenclamide, although the rate of hypoglycaemic episodes tended to be higher in the group receiving insulin than in the glibenclamide group.
The administration of insulin lispro delivered as a continuous subcutaneous insulin infusion (CSII) using an external pump, relative to the administration of insulin lispro as multiple daily injections, improved glycaemic control (and at a lower insulin dose and with fewer hypoglycaemic episodes) in patients with type 1 diabetes, and achieved similar glycaemic control in elderly patients with type 2 diabetes. Comparing insulins delivered as a CSII, insulin lispro achieved similar or better glycaemic control than that achieved with regular human insulin and achieved similar glycaemic control to that achieved with insulin aspart. Insulin lispro as a CSII has also been successfully used in children.
Children receiving insulin lispro achieved postprandial blood glucose levels that were similar to or lower than those achieved with regular human insulin, with similar overall glycaemic control and risk of hypoglycaemia. Adolescents receiving insulin lispro experienced significantly lower postprandial blood glucose levels and a lower rate of hypoglycaemia than recipients of regular human insulin, but overall glycaemic control was similar for both groups.
Prospective and retrospective studies in pregnant women with diabetes indicate that glycaemic control and maternal and fetal outcomes with insulin lispro therapy were generally similar to those with regular human insulin and in line with data available for other insulins.
In large crossover trials, patients with type 1 diabetes using insulin lispro preferred the flexibility and convenience and were more satisfied with their treatment than patients using regular human insulin. Trials in patients with type 2 diabetes indicated that there was no difference in treatment satisfaction between insulin lispro and regular human insulin, but the use of insulin lispro mixtures was preferred over that of glibenclamide.
Tolerability
The tolerability of rapid-acting insulins is essentially concerned with hypoglycaemia. The largest trials in adult patients with type 1 or 2 diabetes receiving insulin lispro demonstrated significantly lower rates of hypoglycaemia, including nocturnal episodes, in insulin lispro recipients than in regular human insulin recipients. In almost all the other trials, the incidence of hypoglycaemia in insulin recipients of all ages was similar to or less than that in regular human insulin or other comparator insulin recipients.







Similar content being viewed by others
Notes
The use of trade names is for identification purposes only and does not imply endorsement.
References
Vajo Z, Fawcett J, Duckworth WC. Recombinant DNA technology in the treatment of diabetes: insulin analogs. Endocr Rev 2001; 22 (5): 706–17
Hirsch I. Insulin analogues. N Engl J Med 2005; 352 (2): 174–83
Ciszak E, Beals JM, Frank BH, et al. Role of C-terminal B-chain residues in insulin assembly: the structure of hexameric LysB28proB29-human insulin. Structure 1995 Jun 15; 3 (6): 615–22
Llewelyn J, Slieker LJ, Zimmermann JL. Preclinical studies on insulin lispro. Drugs Today 1998; 34 Suppl. C: 11–21
Wilde MI, McTavish D. Insulin lispro: a review of its pharmacological properties and therapeutic use in the management of diabetes mellitus. Drugs 1997 Oct; 54: 597–614
Somwar R, Sweeney G, Ramlal T, et al. Stimulation of glucose and amino acid transport and activation of the insulin signaling pathways by insulin lispro in L6 skeletal muscle cells. Clin Ther 1998; 20: 125–40
Kurtzhals P, Schaffer L, Sorensen A, et al. Correlations of receptor binding and metabolic and mitogenic potencies of insulin analogs designed for clinical use. Diabetes 2000 Jun; 49: 999–1005
Eli Lilly and Company. Humalog® (insulin lispro injection): US prescribing information [online]. Available from URL: http://humalog.com [Accessed 2006 Nov 2]
Torlone E, Fanelli C, Rambotti AM, et al. Pharmacokinetics, pharmacodynamics and glucose counterregulation following subcutaneous injection of the monomeric insulin analogue [Lys(B28), Pro(B29)] in IDDM. Diabetologia 1994 Jul; 37 (7): 713–20
Eli Lilly and Company Limited. Humalog® (insulin lispro injection): summary of product characteristics [online]. Available from URL: http://emc.medicines.org.uk [Accessed 2006 Aug 29]
Murase Y, Yagi K, Sugihara M, et al. Lispro is superior to regular insulin in transient intensive insulin therapy in type 2 diabetes. Intern Med 2004 Sep; 43 (9): 779–86
Griffen SC, Oostema K, Stanhope KL, et al. Administration of lispro insulin with meals improves glycemic control, increases circulating leptin, and suppresses ghrelin, compared with regular/NPH insulin in female patients with type 1 diabetes. J Clin Endocrinol Metab 2006 Feb; 91 (2): 485–91
Strachan MW, Frier BM. Optimal time of administration of insulin lispro: importance of meal composition. Diabetes Care 1998 Jan; 21 (1): 26–31
Schernthaner G, Wein W, Sandholzer K, et al. Postprandial insulin lispro: a new therapeutic option for type 1 diabetic patients. Diabetes Care 1998 Apr; 21: 570–3
Rassam AG, Zeise TM, Burge MR, et al. Optimal administration of lispro insulin in hyperglycemic type 1 diabetes. Diabetes Care 1999 Jan; 22: 133–6
Ciofetta M, Lalli C, Del Sindaco P, et al. Contribution of postprandial versus interprandial blood glucose to HbA1c in type 1 diabetes on physiologic intensive therapy with lispro insulin at mealtime. Diabetes Care 1999 May; 22: 795–800
Joseph SE, Korzon-Burakowska A, Woodworth JR, et al. The action profile of lispro is not blunted by mixing in the syringe with NPH insulin. Diabetes Care 1998 Dec; 21: 2098–102
Heise T, Weyer C, Serwas A, et al. Time-action profile of novel premixed preparations of insulin lispro and NPL insulin. Diabetes Care 1998 May; 21: 800–3
Herz M, Arora V, Campaigne BN, et al. Humalog Mix25 improves 24-hour plasma glucose profiles compared with the human insulin mixture 30/70 in patients with type 2 diabetes mellitus. S Afr Med J 2003 Mar; 93: 219–23
Koivisto VA, Tuominen JA, Ebeling P. Lispro Mix25 insulin as premeal therapy in type 2 diabetic patients. Diabetes Care 1999 Mar; 22: 459–62
Malone JK, Yang H, Woodworth JR, et al. Humalog Mix25 offers better mealtime glycemic control in patients with type 1 or type 2 diabetes. Diabetes Metab 2000 Dec; 26 (6): 481–7
Malone JK, Woodworth JR, Arora V, et al. Improved postprandial glycemic control with Humalog Mix75/25 after a standard test meal in patients with type 2 diabetes mellitus. Clin Ther 2000 Feb; 22: 222–30
Garg SK, Anderson JH, Perry SV, et al. Long-term efficacy of Humalog in subjects with type 1 diabetes mellitus. Diabet Med 1999 May; 16: 384–7
McCrimmon RJ, Frier BM. Symptomatic and physiological responses to hypoglycaemia induced by human soluble insulin and the analogue lispro human insulin. Diabet Med 1997 Nov; 14: 929–36
Pfützner A, Küstner E, Forst T, et al. Intensive insulin therapy with insulin lispro in patients with type 1 diabetes reduces the frequency of hypoglycemic episodes. Exp Clin Endocrinol Diabetes 1996; 104 (1): 25–30
Roach P, Strack T, Arora V, et al. Improved glycaemic control with the use of self-prepared mixtures of insulin lispro and insulin lispro protamine suspension in patients with types 1 and 2 diabetes. Int J Clin Pract 2001 Apr; 55: 177–82
Fineberg SE, Huang J, Brunelle R, et al. Effect of long-term exposure to insulin lispro on the induction of antibody response in patients with type 1 or type 2 diabetes. Diabetes Care 2003 Jan; 26: 89–96
Fineberg NS, Fineberg SE, Anderson JH Jr, et al. Immunologic effects of insulin lispro [Lys (B28), Pro (B29) human insulin] in IDDM and NIDDM patients previously treated with insulin. Diabetes 1996 Dec; 45 (12): 1750–4
Recasens M, Aguilera E, Morinígo R, et al. Insulin lispro is as effective as regular insulin in optimising metabolic control and preserving β-cell function at onset of type 1 diabetes mellitus. Diabetes Res Clin Pract 2003 Jun; 60 (3): 153–9
Rave K, Heise T, Weyer C, et al. Intramuscular versus subcutaneous injection of soluble and lispro insulin: comparison of metabolic effects in healthy subjects. Diabet Med 1998 Sep; 15: 747–51
Hoffman A, Ziv E. Pharmacokinetic considerations of new insulin formulations and routes of administration. Clin Pharmacokinet 1997 Oct; 33: 285–301
Czock D, Aisenpreis U, Rasche FM, et al. Pharmacokinetics and pharmacodynamics of lispro-insulin in hemodialysis patients with diabetes mellitus. Int J Clin Pharmacol Ther 2003 Oct; 41 (10): 492–7
Boskovic R, Feig DS, Derewlany L, et al. Transfer of insulin lispro across the human placenta: in vitro perfusion studies. Diabetes Care 2003 May; 26 (5): 1390–4
Holcberg G, Tsadkin-Tamir M, Sapir O, et al. Transfer of insulin lispro across the human placenta [letter]. Eur J Obstet Gynecol Reprod Biol 2004 Jul 15; 115 (1): 117–8
World Health Organisation. Department of Noncommunicable Disease Surveillance. Definition, diagnosis and classification of diabetes mellitus [online]. Available from URL: http://www.who.int [Accessed 2006 Oct 25]
Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997; 20: 1183–97
Rivas M, Acosta A, Roman K. Glycemie excursion with insulin lispro Mix75/25 vs NPH and pen device satisfaction in type 2 diabetes in Puerto Rico [abstract no. 1855-P]. Diabetes 2006 Jun; 55 Suppl. 1: 428
Robbins DC, Beisswenger PJ, Moses RG, et al. Comparison of insulin lispro mid mixture (MM) plus metformin (Met) with glargine (G) plus met on HbA1c (Alc) and blood glucose (BG) profiles in patients with type 2 diabetes (T2D) [abstract no. 0988]. Diabetologia 2006 Sep; 49 Suppl. 1: 603–4
Ghio A, Volpe L, Lencioni C, et al. Safety and efficacy of shortacting insulin aspart and lispro analogues in patients with gestational diabetes mellitus (GDM) [abstract no. 0947]. Diabetologia 2006 Sep; 49 Suppl. 1: 576
Gale EAM. A randomized, controlled trial comparing insulin lispro with human soluble insulin in patients with type 1 diabetes on intensified insulin therapy. UK Trial Group. Diabet Med 2000 Mar; 17: 209–14
Forst T, Eriksson JW, Strotmann HJ, et al. Metabolic effects of mealtime insulin lispro in comparison to glibenclamide in early type 2 diabetes. Exp Clin Endocrinol Diabetes 2003 Apr; 111: 97–103
Anderson JH Jr, Brunelle RL, Koivisto VA, et al. Reduction of postprandial hyperglycemia and frequency of hypoglycemia in IDDM patients on insulin-analog treatment. Multicenter Insulin Lispro Study Group. Diabetes 1997 Feb; 46 (2): 265–70
Anderson JH Jr, Brunelle RL, Koivisto VA, et al. Improved mealtime treatment of diabetes mellitus using an insulin analogue. Multicenter Insulin Lispro Study Group. Clin Ther 1997; 19(1): 62–72
Annuzzi G, Del Prato S, Arcari R, et al. Preprandial combination of lispro and NPH insulin improves overall blood glucose control in type I diabetic patients: a multicenter randomized crossover trial. Nutr Metab Cardiovasc Dis 2001 Jun; 11: 168–75
Heller SR, Amiel SA, Mansell P, et al. Effect of the fast-acting insulin analog lispro on the risk of nocturnal hypoglycemia during intensified insulin therapy. Diabetes Care 1999 Oct; 22: 1607–11
Holleman F, Schmitt H, Rottiers R, et al. Reduced frequency of severe hypoglycemia and coma in well-controlled IDDM patients treated with insulin lispro. Diabetes Care 1997 Dec; 20: 1827–32
Valle D, Santoro D, Bates P, et al. Italian multicentre study of intensive therapy with insulin lispro in 1184 patients with Type 1 diabetes. Diabetes Nutr Metab 2001 Jun; 14: 126–32
Vignati L, Anderson JH Jr, Iversen PW, et al. Efficacy of insulin lispro in combination with NPH human insulin twice per day in patients with insulin-dependent or non-insulin-dependent diabetes mellitus. Clin Ther 1997; 19: 1408–21
Altuntas Y, Ozen B, Ozturk B, et al. Comparison of additional metformin or NPH insulin to mealtime insulin lispro therapy with mealtime human insulin therapy in secondary OAD failure. Diabetes Obes Metab 2003 Nov; 5 (6): 371–8
Bastyr EJ III, Johnson ME, Trautmann ME, et al. Insulin lispro in the treatment of patients with type 2 diabetes mellitus after oral agent failure. Clin Ther 1999 Oct; 21: 1703–14
Bastyr EJ III, Stuart CA, Brodows RG, et al. Therapy focused on lowering postprandial glucose, not fasting glucose, may be superior for lowering HbA1c. Diabetes Care 2000 Sep; 23: 1236–41
Kokic S, Bukovic D, Radman M, et al. Lispro insulin and metformin versus other combination in the diabetes mellitus type 2 management after secondary oral antidiabetic drug failure. Coll Antropol 2003 Jun; 27 (1): 181–7
Ross SA, Zinman B, Campos RV, et al. A comparative study of insulin lispro and human regular insulin in patients with type 2 diabetes mellitus and secondary failure of oral hypoglycemic agents. Clin Invest Med 2001 Dec; 24: 292–8
Anderson JH Jr, Brunelle RL, Keohane P, et al. Mealtime treatment with insulin analog improves postprandial hyperglycemia and hypoglycemia in patients with non-insulin-dependent diabetes mellitus. Multicenter Insulin Lispro Study Group. Arch Intern Med 1997 Jun 9; 157 (11): 1249–55
Herz M, Arora V, Sun B, et al. Basal-bolus insulin therapy in Type 1 diabetes: comparative study of pre-meal administration of a fixed mixture of insulin lispro (50%) and neutral protamine lispro (50%) with human soluble insulin. Diabet Med 2002 Nov; 19: 917–23
Roach P, Bai S, Charbonnel B, et al. Effects of multiple daily injection therapy with Humalog mixtures versus separately injected insulin lispro and NPH insulin in adults with type I diabetes mellitus. Clin Ther 2004 Apr; 26 (4): 502–10
Roach P, Trautmann M, Arora V, et al. Improved postprandial blood glucose control and reduced nocturnal hypoglycemia during treatment with two novel insulin lispro-protamine formulations, insulin lispro Mix25 and insulin lispro Mix 50. Clin Ther 1999 Mar; 21: 523–34
Galic E, Vrtovec M, Bozikov V, et al. The impact of the timing of Humalog Mix25 injections on blood glucose fluctuations in the postprandial period in elderly patients with type 2 diabetes. Med Sci Monit 2005 Dec; 11 (12): PI87–92
Herz M, Sun B, Milicevic Z, et al. Comparative efficacy of preprandial or postprandial Humalog Mix75/25 versus glyburide in patients 60 to 80 years of age with type 2 diabetes mellitus. Clin Ther 2002 Jan; 24: 73–86
Roach P, Koledova E, Metcalfe S, et al. Glycemie control with Humalog Mix25 in type 2 diabetes inadequately controlled with glyburide. Clin Ther 2001 Oct; 23: 1732–44
Niskanen L, Jensen LE, Rastam J, et al. Randomized, multinational, open-label, 2-period, crossover comparison of biphasic insulin aspart 30 and biphasic insulin lispro 25 and pen devices in adult patients with type 2 diabetes mellitus. Clin Ther 2004 Apr; 26 (4): 531–40
Roach P, Yue L, Arora V, et al. Improved postprandial glycemic control during treatment with Humalog Mix25, or a novel protamine-based insulin lispro formulation. Diabetes Care 1999 Aug; 22: 1258–61
Malone JK, Bai S, Campaigne BN, et al. Twice-daily pre-mixed insulin rather than basal insulin therapy alone results in better overall glycaemic control in patients with Type 2 diabetes. Diabet Med 2005 Apr; 22 (4): 374–81
Kazda C, Hulstrunk H, Helsberg K, et al. Prandial insulin substitution with insulin lispro or insulin lispro mid mixture vs. basal therapy with insulin glargine: a randomized controlled trial in patients with type 2 diabetes beginning insulin therapy. J Diabetes Complications 2006; 20 (3): 145–52
Roach P, Arora V, Campaigne BN, et al. Humalog Mix50 before carbohydrate-rich meals in type 2 diabetes mellitus. Diabetes Obes Metab 2003 Sep; 5 (5): 311–6
Bode B, Weinstein R, Bell D, et al. Comparison of insulin aspart with buffered regular insulin and insulin lispro in continuous subcutaneous insulin infusion: a randomised study in type 1 diabetes. Diabetes Care 2002 Mar; 25: 439–44
Hoogma RPLM, Hammond PJ, Gomis R, et al. Comparison of the effects of continuous subcutaneous insulin infusion (CSII) and NPH-based multiple daily insulin injections (MDI) on glycaemic control and quality of life: results of the 5-nations trial. Diabet Med 2006 Feb; 23 (2): 141–7
Renner R, Pfützner A, Trautmann M, et al. Use of insulin lispro in continuous subcutaneous insulin infusion treatment: results of a multicenter trial. Diabetes Care 1999 May; 22: 784–8
Herman WH, Ilag LL, Johnson SL, et al. A clinical trial of continuous subcutaneous insulin infusion versus multiple daily injections in older adults with type 2 diabetes. Diabetes Care 2005 Jul; 28 (7): 1568–73
Deeb LC, Holcombe JH, Brunelle R, et al. Insulin lispro lowers postprandial glucose in prepubertal children with diabetes. Pediatrics 2001 Nov; 108: 1175–9
Fairchild JM, Ambler GR, Genoud-Lawton CH, et al. Insulin lispro versus regular insulin in children with type 1 diabetes on twice daily insulin. Pediatr Diabetes 2000 Sep; 1 (3): 135–41
Holcombe JH, Zalani S, Arora VK, et al. Comparison of insulin lispro with regular human insulin for the treatment of type 1 diabetes in adolescents. Clin Ther 2002 Apr; 24: 629–38
Delia Manna T, Steinmetz L, Campos PR, et al. Subcutaneous use of a fast-acting insulin analog: an alternative treatment for pediatric patients with diabetic ketoacidosis. Diabetes Care 2005 Aug; 28 (8): 1856–61
Maniatis AK, Klingensmith GJ, Slover RH, et al. Continuous subcutaneous insulin infusion therapy for children and adolescents: an option for routine diabetes care. Pediatrics 2001 Feb; 107: 351–6
Weintrob N, Benzaquen H, Galatzer A, et al. Comparison of continuous subcutaneous insulin infusion and multiple daily injection regimens in children with type 1 diabetes: a randomized open crossover trial. Pediatrics 2003 Sep 1; 112 (3): 559–64
Tubiana-Rufi N, Coutant R, Bloch J, et al. Special management of insulin lispro in continuous subcutaneous insulin infusion in young diabetic children: a randomized cross-over study. Horm Res 2004; 62 (6): 265–71
Loukovaara S, Immonen I, Teramo KA, et al. Progression of retinopathy during pregnancy in type 1 diabetic women treated with insulin lispro. Diabetes Care 2003 Apr; 26: 1193–8
Cypryk K, Sobczak M, Pertyńska-Marczewska M, et al. Pregnancy complications and perinatal outcome in diabetic women treated with Humalog (insulin lispro) or regular human insulin during pregnancy. Med Sci Monit 2004 Feb; 10 (2): P129–32
Mecacci F, Carignani L, Cioni R, et al. Maternal metabolic control and perinatal outcome in women with gestational diabetes treated with regular or lispro insulin: comparison with non-diabetic pregnant women. Eur J Obstet Gynecol Reprod Biol 2003 Nov 10; 111 (1): 19–24
Garg SK, Frias JP, Anil S, et al. Insulin lispro therapy in pregnancies complicated by type 1 diabetes: glycemic control and maternal and fetal outcomes. Endocr Pract 2003; 9(3): 187–93
Masson EA, Patmore JE, Brash PD, et al. Pregnancy outcome in type 1 diabetes mellitus treated with insulin lispro (Humalog). Diabet Med 2003 Jan; 20: 46–50
Mattoo V, Milicevic Z, Malone JK, et al. A comparison of insulin lispro Mix25 and human insulin 30/70 in the treatment of type 2 diabetes during Ramadan. Diabetes Res Clin Pract 2003 Feb; 59: 137–43
Kotsanos JG, Vignati L, Huster W, et al. Health-related qualityof-life results from multinational clinical trials of insulin lispro: assessing benefits of a new diabetes therapy. Diabetes Care 1997 Jun; 20 (6): 948–58
Brunelle RL, Llewelyn J, Anderson JH Jr, et al. Meta-analysis of the effect of insulin lispro on severe hypoglycemia in patients with type 1 diabetes. Diabetes Care 1998 Oct; 21: 1726–31
Glazer NB, Zalani S, Anderson JH Jr, et al. Safety of insulin lispro: pooled data from clinical trials. Am J Health Syst Pharm 1999 Mar 15; 56: 542–7
Bastyr EJ III, Kotsanos J, Vignati L, et al. Insulin lispro (LP) reduces hypoglycemia rate in persons with type II diabetes at high risk for hypoglycemia [abstract no. 202]. Diabetes 1996 May; 45 Suppl. 2: 56
Wyatt JW, Frias JL, Hoyme HE, et al. Congenital anomaly rate in offspring of mothers with diabetes treated with insulin lispro during pregnancy. Diabet Med 2005 Jun; 22 (6): 803–7
Bhattacharyya A, Brown S, Hughes S, et al. Insulin lispro and regular insulin in pregnancy. QJM 2001; 94: 255–60
European Diabetes Policy Group 1999. A desktop guide to type 2 diabetes mellitus. Diabet Med 1999; 16 (9): 716–30
European Diabetes Policy Group 1998. A desktop guide to type 1 (insulin-dependent) diabetes mellitus. Diabet Med 1999; 16 (3): 253–66
American Diabetes Association. Standards of medical care in diabetes: 2006. Diabetes Care 2006 Jan; 29 Suppl. 1: S4–42
Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993 Sep; 329(14): 977–86
UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998 Sep; 352: 837–53
Oiknine R, Bernbaum M, Mooradian AD. A critical appraisal of the role of insulin analogues in the management of diabetes mellitus. Drugs 2005; 65 (3): 325–40
DECODE Study Group. Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria. European Diabetes Epidemiology Group. Lancet 1999 Aug 21; 354: 617–21
Chapman TM, Noble S, Goa KL. Insulin aspart: a review of its use in the management of type 1 and 2 diabetes mellitus. Drugs 2002; 62 (13): 1945–81
Robinson DM, Wellington K. Insulin glulisine. Drugs 2006; 66 (6): 861–9
Heller S. Insulin lispro: a useful advance in insulin therapy. Expert Opin Pharmacother 2003; 4 (8): 1407–16
Chase HP, Lockspeiser T, Peery B, et al. The impact of the Diabetes Control and Complications Trial and Humalog insulin on glycohemoglobin levels and severe hypoglycemia in type 1 diabetes. Diabetes Care 2001 Mar; 24: 430–4
Del Sindaco P, Ciofetta M, Lalli C, et al. Use of the short-acting insulin analogue lispro in intensive treatment of type 1 diabetes mellitus: importance of appropriate replacement of basal insulin and time-interval injection-meal. Diabet Med 1998 Jul; 15 (7): 592–600
Rossetti P, Pampanelli S, Fanelli C, et al. Intensive replacement of basal insulin in patients with type 1 diabetes given rapid acting insulin analogue at mealtime. Diabetes Care 2003; 26 (1): 1490–6
Ristic S, Bates PC. Effects of rapid acting insulin analogues on overall glycemic control in type 1 and type 2 diabetes mellitus. Diabetes Technol Ther 2003; 5 (1): 57–66
Garber AJ. Premixed insulin analogues for the treatment of diabetes mellitus. Drugs 2006; 66 (1): 31–49
Coscelli C, Iacobellis G, Calderini C. Importance of premeal injection time in insulin therapy: Humalog Mix25 is convenient for improved post-prandial glycemic control in type 2 diabetic patients with Italian dietary habits. Acta Diabetol 2003; 40 (4): 187–92
White NH, Cleary PA, Dahms W, et al. Beneficial effects of intensive therapy of diabetes during adolescence: outcomes after the conclusion of the Diabetes Control and Complications Trial (DCCT). J Paediatr 2001; 139(6): 804–12
Carr KJ, Idama TO, Masson EA, et al. A randomised controlled trial of insulin lispro given before or after meals in pregnant women with type 1 diabetes: the effect on glycaemic excursion. J Obstet Gynaecol 2004 Jun; 24 (4): 382–6
Gottlieb PA, Frias JP, Peters KA, et al. Optimizing insulin therapy in pregnant women with type 1 diabetes mellitus. Treat Endocrinol 2002; 1 (4): 235–40
Carr KJ, Lindow SW, Masson EA. The potential for the use of insulin lispro in pregnancy complicated by diabetes. J Matern Fetal Neonatal Med 2006 Jun; 19(6): 323–9
Buchbinder A, Miodovnik M, Khoury J, et al. Is the use of insulin lispro safe in pregnancy? J Matern Fetal Neonatal Med 2002 Apr; 11 (4): 232–7
Castéra V, Dutour-Meyer A, Koeppel MC, et al. Systemic allergy to human insulin and its rapid and long acting analogs: successful treatment by continuous subcutaneous insulin lispro infusion. Diabetes Metab 2005 Sep; 31 (4): 391–400
Dunn CJ, Plosker GL. Insulin lispro: a pharmacoeconomic review of its use in diabetes mellitus. Pharmacoeconomics 2002; 20 (14): 989–1025
Author information
Authors and Affiliations
Additional information
Various sections of the manuscript reviewed by: K. Cypryk, Clinic of Endocrinology and Metabolic Diseases, Medical University of Lodz, Lodz, Poland; G. Forlani, Metabolic Diseases Unit, Department of Internal Medicine and Gastroenterology, University of Bologna, Bologna, Italy; D.R. Hadden, Department of Endocrinology, Royal Victoria Hospital, Belfast, Northern Ireland; H. Lawall, Klinikum Karlsbad-Langensteinbach, Karlsbad, Germany; G.D. Lopaschuk, Cardiovascular Research Group, University of Alberta, Edmonton, Alberta, Canada; G. Tamas, Diabetes Unit, Department of Medicine, Semmelweis University, Budapest, Hungary.
Data Selection
Sources: Medical literature published in any language since 1980 on ‘insulin lispro’, identified using MEDLINE and EMBASE, supplemented by AdisBase (a proprietary database of Wolters Kluwer Health | Adis). Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: MEDLINE, EMBASE and AdisBase search terms were ‘insulin lispro’. Searches were last updated 7 February 2007.
Selection: Studies in patients with who received insulin lispro. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Insulin lispro, human insulin analogue, diabetes mellitus, pharmacodynamics, pharmacokinetics, therapeutic use, tolerability.
Rights and permissions
About this article
Cite this article
Simpson, D., McCormack, P.L., Keating, G.M. et al. Insulin Lispro. Drugs 67, 407–434 (2007). https://doi.org/10.2165/00003495-200767030-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200767030-00006