Abstract
-
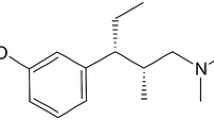
▴ Tramadol is a synthetic, centrally acting opioid analgesic. An extended-release tablet formulation of tramadol (tramadol ER) allows gradual release of the active drug, permitting once-daily administration.
-
▴ Tramadol ER administered once daily is equivalent in bioavailability to immediate-release tramadol administered four times daily, with prolonged absorption and lower peak plasma concentrations.
-
▴ Tramadol ER was significantly more effective than placebo in the treatment of moderate to moderately severe chronic pain in patients with osteoarthritis of the knee and/or hip in randomised, double-blind, placebo-controlled trials. In a flexible-dose trial in patients with osteoarthritis of the knee, the mean reduction from baseline in pain intensity scores over 12 weeks was significantly greater in recipients of tramadol ER than in placebo recipients.
-
▴ In a fixed-dose trial in patients with osteoarthritis of the knee and/or hip, the mean improvements from baseline in the pain and physical function subscale scores of the Western Ontario and McMaster Universities Osteoarthritis Index over 12 weeks were significantly greater in tramadol ER than placebo recipients.
-
▴ Common adverse events reported in patients with moderate to moderately severe chronic pain treated with tramadol ER 100–300mg once daily were dizziness (excluding vertigo), nausea, constipation, somnolence and flushing.






Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Best Practice Committee of the Health Care Association of New Jersey. Hamilton (NJ). Pain management guideline [online]. Available from URL: http://www.hcanj.org/docs/hcanjbp_painmgmt2.pdf [Accessed 2006 Oct 4]
Wisconsin Medical Society Task Force on Pain Management. Guidelines for the assessment and management of chronic pain. Wis Med J 2004; 103(3): 15–42
ULTRAM® (tramadol hydrochloride tablets). US prescribing information May 2004. Ortho-McNeil Pharmaceutical Inc., Raritan (NJ) [online]. Available from URL: http://www.orthomcneil.com [Accessed 2006 Oct 2]
Grond S, Sablotzki A. Clinical pharmacology of tramadol. Clin Pharmacokinet 2004; 43(13): 879–923
Keating GM. Tramadol sustained-release capsules. Drugs 2006; 66(2): 223–30
Mattia C, Coluzzi F. Once-daily tramadol in rheumatological pain. Expert Opin Pharmacother 2006; 7(13): 1811–23
ULTRAM® ER (tramadol HCl) extended-release tablets: an oral once-daily formulation of tramadol. Formulary 2006 Sep; 41 Suppl.: 1-17
Biovail Corporation. Investor conference: June 28, 2005 [online]. Available from URL: http://www.biovail.com/local/files/Commercial%20Operations.pdf [Accessed 2006 Sep 29]
Modified release formulations of at least one form of tramadol. United States patent application: 20060099249. Kind code: A 1. Inventors: Seth, Pawan, et al. May 11, 2006 [online]. Available from URL: http://www.uspto.gov [Accessed 2006 Oct 1]
Biovail Corporation. Biovail Corp International 20-F for 12/31/05 [online]. Available from URL: http://www.secinfo.com/dVut2.v49s.htm [Accessed 2006 Sep 24]
ULTRAM® ER (tramadol HCl) extended-release tablets. US prescribing information January 2006. Ortho-McNeil Inc., Raritan (NJ) [online]. Available from URL: http://www.orthomcneil.com [Accessed 2006 Oct 2]
Scott LJ, Perry CM. Tramadol: a review of its use in perioperative pain. Drugs 2000; 60(1): 139–76
Eradiri O, Sista S, Lai JC-K, et al. Bioavailability of extended-release and immediate-release formulations of tramadol HCl [abstract no. 126]. J Clin Pharmacol 2006; 46(9): 1091. Plus poster presented at the 35th Annual Meeting of the American College of Clinical Pharmacology; 2006 Sep 17–19; Cambridge (MA)
Lai JC-K, Sista S, Eradiri O, et al. Influence of CYP2D6 inhibition on pharmacokinetics of tramadol HCl extended release tablets in normal healthy subjects [abstract no. 128]. J Clin Pharmacol 2006; 46(9): 1092. Plus poster presented at the 35th Annual Meeting of the American College of Clinical Pharmacology; 2006 Sep 17–19; Cambridge (MA)
Lai JC, Sista S, Eradiri O, et al. The pharmacokinetics of novel once daily tramadol hydrochloride extended release tablets in healthy subjects [abstract no. M1071]. AAPS PharmSci 2003; 5 Suppl. 1. Plus poster presented at the Annual Meeting and Exposition of the American Association of Pharmaceutical Scientists; 2003 Oct 26–30; Salt Lake City (UT)
Sista S, Lai JC, Eradiri O, et al. Steady state dose proportionality of a novel formulation of tramadol HCl extended release tablets [abstract no. M1076]. AAPS PharmSci 2003; 5 Suppl. 1. Plus poster presented at the Annual Meeting and Exposition of the American Association of Pharmaceutical Scientists; 2003 Oct 26–30; Salt Lake City (UT)
Lai JC-K, Sista S, Eradiri O, et al. Steady-state pharmacokinetics of tramadol HCl extended-release tablets in patients with mild and moderate renal failure [abstract no. 129]. J Clin Pharmacol 2006; 46(9): 1092. Plus poster presented at the 35th Annual Meeting of the American College of Clinical Pharmacology; 2006 Sep 17–19; Cambridge (MA)
Eradiri O, Sista S, Danyluk A, et al. Stereospecific pharmacokinetics of tramadol HCl extended release tablets in patients with mild and moderate hepatic impairment [abstract no. 127]. J Clin Pharmacol 2006; 46(9): 1091. Plus poster presented at the 35th Annual Meeting of the American College of Clinical Pharmacology; 2006 Sep 17–19; Cambridge (MA)
Babul N, Noveck R, Chipman H, et al. Efficacy and safety of extended-release, once-daily tramadol in chronic pain: a randomized 12-week clinical trial in osteoarthritis of the knee. J Pain Symptom Manage 2004; 28(1): 59–71
Gana TJ, Pascual MLG, Fleming RRB, et al. Extended-release tramadol in the treatment of osteoarthritis: a multicenter, randomized, double-blind, placebo-controlled clinical trial. 023 Study Group. Curr Med Res Opin 2006; 22(7): 1391–401
Janagap C, Schein J, Kosinski M, et al. Extended-release tramadol improves sleep in osteoarthritis [abstract plus poster]. 17th Annual Clinical Meeting of the American Academy of Pain Management; 2006 Sep 7–10; Orlando (FL), 47-8
Vorsanger G, Jordan D, Xiang J, et al. Effect of extended-release tramadol on pain in the elderly [abstract plus poster]. 17th Annual Clinical Meeting of the American Academy of Pain Management; 2006 Sep 7–10; Orlando (FL), 57
Danyluk AP, Murthy BP, Skee DM, et al. Dose conversion for immediate- to extended-release tramadol [abstract plus poster]. 17th Annual Clinical Meeting of the American Academy of Pain Management; 2006 Sep 7–10; Orlando (FL), 46
World Health Organization. WHO’s pain ladder [online]. Available from URL: http://www.who.int/cancer/palliative/painladder/en [Accessed 2006 Sep 19]
American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. Arthritis Rheum 2000; 43(9): 1905–15
Cashman JN. Current pharmacotherapeutic strategies in rheumatic diseases and other pain states. Clin Drug Invest 2000; 19 Suppl. 2: 9–20
Lee CR, McTavish D, Sorkin EM. Tramadol: a preliminary review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in acute and chronic pain states. Drugs 1993; 46(2): 313–40
Boehringer Ingelheim Pharmaceuticals Inc. Ridgefield (CT). Mobic® (meloxicam). US prescribing information [online]. Available from URL: http://us.boehringer-ingelheim.com [Accessed 2006 Oct 2]
Roche Laboratories Inc. Nutley (NJ). NAPROSYN® (naproxen) tablets. US prescribing information [online]. Available from URL: http://www.rocheusa.com [Accessed 2006 Oct 2]
Novartis Pharmaceuticals Corporation. East Hanover (NJ). Voltaren® (diclofenac sodium enteric-coated tablets). US prescribing information [online]. Available from URL: http://www.pharma.us.novartis.com [Accessed 2006 Oct 2]
Pfizer Inc. New York (NY). CELEBREX® celecoxib capsules. US prescribing information [online]. Available from URL: http://www.celebrex.com/information/Celebrex_PI.pdf [Accessed 2006 Oct 2]
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: M.P. Davis, Harry R. Horvitz Center for Palliative Medicine, Cleveland Clinic Foundation, Cleveland, Ohio, USA; B.H. McCarberg, Kaiser Permanente, Escondido, California, USA; C.J. Sachs, UCLA Emergency Medicine Center, Los Angeles, California, USA.
Rights and permissions
About this article
Cite this article
Hair, P.I., Curran, M.P. & Keam, S.J. Tramadol Extended-Release Tablets. Drugs 66, 2017–2027 (2006). https://doi.org/10.2165/00003495-200666150-00014
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200666150-00014