Abstract
-
▴ The methylphenidate transdermal system (MTS) patch is approved by the US FDA for use in children aged 6–12 years with attention-deficit hyperactivity disorder (ADHD). This delivery system permits sustained absorption of the drug through the skin and into the bloodstream. Methylphenidate (MPH) is a CNS agent thought to act on dopamine and noradrenaline (norepinephrine) pathways and thereby blocks the reuptake of these neurotransmitters into the presynaptic neuron.
-
▴ In children with ADHD, MTS patches releasing MPH doses of 10–30mg over a 9-hour period (12.5–37.5cm2 patch size) is steadily absorbed, with mean peak plasma concentrations of d-MPH (20–46.5 ng/mL) reached in ≊8 hours.
-
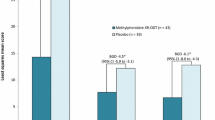
▴ In well controlled trials in children with ADHD, patients administered MTS patches releasing MPH 10–30mg over ≊9 hours showed significantly greater improvements in their ADHD symptoms than placebo recipients.
-
▴ MTS patches are generally well tolerated in paediatric patients with ADHD, with treatment-emergent events being similar in nature to those reported with oral MPH. The majority of adverse events were mild to moderate in intensity.






Similar content being viewed by others
Notes
Daytrana is a trademark of Shire Pharmaceuticals Ireland Limited. The use of trade names is for product identification purposes only and does not imply endorsement.
DOT Matrix is a trademark of Noven Pharmaceuticals, Inc.
OROS® is a trademark of ALZA Corporation.
References
American Academy of Pediatrics. Clinical practice guideline: diagnosis and evaluation of the child with attention-deficit/ hyperactivity disorder. Pediatrics 2000; 105: 1158–70
Findling RL, Dogin JW. Psychopharmacology of ADHD: children and adolescents. J Clin Psychiatry 1998; 59 Suppl. 7: 42–9
Greenhill LL, Halperin JM, Abikoff H. Stimulant medications. J Am Acad Child Adolesc Psychiatry 1999 May; 38(5): 503–12
Noven Pharmaceuticals Inc. Noven’s DOT Matrix® technology [online]. Available from URL: http://www.noven.com/research.htm [Accessed 2005 Dec 19]
Shire Pharmaceuticals Ireland Limited. Daytrana™ (methylphenidate transdermal system) [online]. Available from URL: http://www.daytrana.com [Accessed 2006 Apr 7]
Kimko HC, Cross JT, Abernethy DR. Pharmacokinetics and clinical effectiveness of methylphenidate. Clin Pharmacokinet 1999 Dec; 37(6): 457–70
Markowitz JS, Patrick KS. Pharmacokinetic and pharmacodynamic drug interactions in the treatment of attention-deficit hyperactivity disorder. Clin Pharmacokinet 2001; 40(10): 753–72
Rapport MD, Moffitt C. Attention deficit/hyperactivity disorder and methylphenidate. A review of height/weight, cardiovascular, and somatic complaint side effects. Clin Psychol Rev 2002 Nov; 22(8): 1107–31
Lyseng-Williamson KA, Keating GM. Extended-release methylphenidate (Ritalin® LA). Drugs 2002; 62(15): 2251–9
Novartis. Ritalin LA® (methylphenidate hydrochloride) extended-release capsules: prescribing information [online]. Available from URL: http://www.pharma.us.novartis.com [Accessed 2006 May 26]
Pelham WE, Manos MJ, Ezzell CE, et al. A dose-ranging study of a methylphenidate transdermal system in children with ADHD. J Am Acad Child Adolesc Psychiatry 2005 Jun; 44(6): 522–9
American Academy of Pediatrics. National institute of mental health multimodal treatment study of ADHD follow-up: changes in effectiveness and growth after the end of treatment. Pediatrics 2004; 113: 762–9
Data on file. Shire, 2006 Feb 10
Wigal SB, Pierce DM, Dixon CM, et al. Pharmacokinetics of methylphenidate transdermal system in children with ADHD [poster no. 207]. 18th Annual U.S. Psychiatric and Mental Health Congress; 2005 Nov 7–10; Las Vegas (NV)
Gonzalez MA. Methylphenidate pharmacokinetics after dosing with a once-daily transdermal system [abstract no. 57A]. 48th Annual Meeting American Academy of Child and Adolescent Psychiatry; 2001 Oct 23–28; Honolulu (HI), 97
Duchin K, Campbell DA, Linares E. Pharmacokinetics of multiple doses of methylphenidate transdermal system in children with attention deficit hyperactivity disorder [poster]. 49th Annual Meeting of the American Academy of Child and Adolescent Psychiatry; 2002 Oct 22–27; San Francisco (CA)
McGough JJ, Wigal SB, Abikoff H, et al. A randomized, double-blind, placebo-controlled, laboratory classroom assessment of methylphenidate transdermal system in children with ADHD. J Atten Disord 2006 Feb; 9(3): 476–85
Melmed R, Findling RL, Lopez FA. The effects of transdermal methylphenidate with reference to OROS methylphenidate in ADHD [poster no. 205]. 18th Annual U.S. Psychiatric and Mental Health Congress; 2005 Nov 7–10; Las Vegas (NV)
Greenhill LL, Pelham W, Lopez F, et al. Once-daily transdermal methylphenidate improves teacher, parent, and CGI-I ratings [abstract no. A27 plus poster]. 49th Annual Meeting of the American Academy of Child and Adolescent Psychiatry; 2002 Oct 22–27; San Francisco (CA), 93–4
Pelham WE, Burrows-MacLean L, Gnagy EM, et al. Transdermal methylphenidate, behavioral, and combined treatment for children with ADHD. Exp Clin Psychopharmacol 2005 May; 13(2): 111–26
Noven Pharmaceuticals I. Safety summary. NDA NO: 21-514 [online]. Available from URL: http://www.fda.gov [Accessed 2006 May 19]
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Anderson, V.R., Scott, L.J. Methylphenidate Transdermal System. Drugs 66, 1117–1126 (2006). https://doi.org/10.2165/00003495-200666080-00007
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200666080-00007