Summary
Abstract
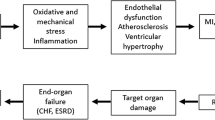
Perindopril (Coversyl®) is a prodrug ester of perindoprilat, an ACE inhibitor. This agent has shown pharmacodynamic effects beyond those responsible for lowering blood pressure (BP), including the improvement of endothelial function and the normalisation of vascular and cardiac structure and function.
Perindopril has a well established role in the treatment of patients with hypertension or heart failure. In the EUROPA trial, once-daily perindopril 8mg prevented cardiovascular events in patients with stable coronary artery disease (CAD) without any apparent heart failure receiving standard recommended therapy. In the ASCOT-BPLA trial, a calcium channel antagonist ± perindopril regimen demonstrated significant cardiovascular benefits compared with a conventional β-blocker ± diuretic regimen in patients with hypertension who were at risk of developing cardiovascular events. These trials demonstrate that while perindopril, in addition to standard recommended therapy, has a potential role in preventing cardiovascular events in hypertensive patients, its role in the management of patients with stable CAD is clearly established.
Pharmacological Properties
Perindopril is a prodrug ester of perindoprilat, an ACE inhibitor. This agent demonstrates pharmacodynamic effects beyond those responsible for lowering BP involving the improvement of endothelial dysfunction and reduction in markers of inflammation and thrombosis. In the PERTINENT trial, perindopril significantly increased plasma levels of bradykinin, and improved endothelial cell nitric oxide synthetase activity. Perindopril reduced levels of angiotensin II, D-dimer and von Willebrand factor and reduced the rate of endothelial apoptosis.
Perindopril demonstrated anti-remodelling effects in small resistance arteries, large arteries and in the heart. Perindopril reduced the media thickness-to-lumen diameter ratio of small arteries and reduced peripheral vascular resistance in patients with hypertension. In small coronary arterioles, the reduction in periarteriolar collagen area and total interstitial collagen volume density in perindopril recipients was associated with a significant increase in coronary blood flow and in coronary reserve. Long-term treatment with perindopril reduced carotid and radial artery wall hypertrophy. In patients with end-stage renal disease, perindopril, independent of BP changes, reduced aortic pulse wave velocity. In the heart, perindopril significantly reduced left ventricular mass in patients with hypertension.
Following oral administration, perindopril is rapidly absorbed, with peak plasma concentrations being reached within 1 hour. Approximately 20–50% of the perindopril absorbed is converted to the active metabolite perindoprilat. Peak plasma concentrations of perindoprilat occur after 3–7 hours; protein binding of perindoprilat is 10–20%. The apparent mean half-life for the majority of perindoprilat elimination is about 3–10 hours, but perindoprilat has a prolonged terminal elimination half-life of 30–120 hours, reflecting slow dissociation from ACE binding sites. Perindoprilat is eliminated in the urine. Food intake may reduce biotransformation of perindopril to perindoprilat.
Therapeutic Efficacy
In the randomised, double-blind EUROPA trial, 12 218 patients with stable CAD and without heart failure were randomised to once-daily perindopril 8mg or placebo. After a mean follow-up of 4.2 years, a significant (p = 0.0003) relative risk reduction (RRR) of 20% for the primary endpoint (cardiovascular death, nonfatal myocardial infarction [MI] and resuscitated cardiac arrest) occurred with perindopril versus placebo. This benefit was consistent in predefined subgroups and was irrespective of treatment with other standard recommended therapy, including platelet inhibitors, lipid-lowering agents and β-blockers.
In the 12-month, randomised, double-blind PREAMI trial in 1252 elderly patients with recent MI and preserved left ventricular ejection fraction, once-daily perindopril 8mg, compared with placebo, was associated with a significant RRR of 38% (p < 0.001) for the primary endpoint (death, hospitalisation for heart failure and cardiac remodelling). Significantly less left ventricular remodelling occurred in perindopril recipients than in placebo recipients (27.7% vs 51.2%, RRR 46%; p < 0.001).
In the randomised, double-blind PROGRESS study in 6105 patients with a previous history of stroke or transient ischaemic attack, the risk of stroke was 10% in recipients of perindopril ± indapamide and 14% in placebo recipients (RRR 28%; p < 0.0001) over a mean follow-up of 3.9 years. BP was reduced by 9/ 4mm Hg in recipients of perindopril ± indapamide compared with placebo. Perindopril ± indapamide reduced the risk of nonfatal myocardial infarction by 38% (95% CI 14, 55) and congestive heart failure by 26% (p = 0.02).
The randomised, open-label ASCOT-BPLA trial in 19 257 hypertensive patients at moderate risk of developing cardiovascular events was terminated prematurely (median follow-up of 5.5 years) because the risk reduction of all-cause mortality was 11 % lower in those treated with amlodipine ± perindopril than in those treated with atenolol ± thiazide. The RRR of 10% for the primary endpoint (nonfatal MI and fatal CHD) in recipients of amlodipine ± perindopril was not significant; however, because of the early termination of the trial, the study was not sufficiently powered for this endpoint.
Tolerability
Perindopril is generally well tolerated in patients with hypertension, heart failure or CAD. Generally, adverse events were mild and transient, with cough, gastrointestinal disturbances and asthenia/fatigue (all <10%) being most commonly reported in a large (n = 47 351) postmarketing surveillance study in patients with hypertension. In the EUROPA trial in patients with CAD, withdrawals in perindopril and placebo recipients were for cough (2.7% vs 0.5%), hypotension (1.0% vs 0.3%) or drug intolerance (2.4% vs 1.3%).





Similar content being viewed by others
Notes
The use of trade names is for identification purposes only and does not imply endorsement.
References
Lloyd-Jones DM, Larson MG, Beiser A, et al. Lifetime risk of developing coronary heart disease. Lancet 1999 Jan 9; 353(9147): 89–92
Bertrand ME. Provision of cardiovascular protection by ACE inhibitors: a review of recent trials. Curr Med Res Opin 2004 Oct; 20(10): 1559–69
Hurst M, Jarvis B. Perindopril: an updated review of its use in hypertension. Drugs 2001; 61(6): 867–96
Simpson D, Noble S, Goa K. Perindopril: in congestive heart failure. Drugs 2002; 62(9): 1367–77
Zhuo JL, Froomes P, Casley D, et al. Perindopril chronically inhibits angiotensin-converting enzyme in both the endothelium and adventitia of the internal mammary artery in patients with ischemic heart disease. Circulation 1997 Jul 1; 96(1): 174–82
Bussien JP, d’Amore TF, Perret L, et al. Single and repeated dosing of the converting enzyme inhibitor perindopril to normal subjects. Clin Pharmacol Ther 1986 May; 39(5): 554–8
Louis WJ, Workman BS, Conway EL, et al. Single-dose and steady-state pharmacokinetics and pharmacodynamics of perindopril in hypertensive subjects. J Cardiovasc Pharmacol 1992 Sep; 20(3): 505–11
Zhuo JL, Mendelsohn FA, Ohishi M. Perindopril alters vascular angiotensin-converting enzyme, AT(1) receptor, and nitric oxide synthase expression in patients with coronary heart disease. Hypertension 2002 Feb; 39 (2 Pt 2): 634–8
Dzau VJ, Bernstein K, Celermajer D, et al. The relevance of tissue angiotensin-converting enzyme: manifestations in mechanistic and endpoint data. Am J Cardiol 2001 Nov 8; 88(9A): 1–20L
Morishita T, Tsutsui M, Shimokawa H, et al. Long-term treatment with perindopril ameliorates dobutamine-induced myocardial ischemia in patients with coronary artery disease. Jpn J Pharmacol 2002 Jan; 88: 100–7
Candido R, Jandeleit-Dahm KA, Cao Z, et al. Prevention of accelerated atherosclerosis by angiotensin-converting enzyme inhibition in diabetic apolipoprotein E-deficient mice. Circulation 2002 Jul 9; 106(2): 246–53
Fennessy PA, Campbell JH, Mendelsohn FA, et al. Angiotensinconverting enzyme inhibitors and atherosclerosis: relevance of animal models to human disease. Clin Exp Pharmacol Physiol 1996 Aug; 23(8): S30–32
Ceconi C, Fox KM, Remme WJ, et al. Effect of perindopril in patients with stable coronary artery disease: results of the PERTINENT Sub-Study [abstract no.3665]. J Hypertens 2005 Jun; 23 Suppl. 2: 274
Fogari R, Mugellini A, Zoppi A, et al. Losartan and perindopril effects on plasma plasminogen activator inhibitor-1 and fibrinogen in hypertensive type 2 diabetic patients. Am J Hypertens 2002 Apr; 15 (4 Pt 1): 316–20
Kishi Y, Ohta S, Kasuya N, et al. Perindopril augments ecto-ATP diphosphohydrolase activity and enhances endothelial anti-platelet function in human umbilical vein endothelial cells. J Hypertens 2003 Jul; 21(7): 1347–53
Antony I, Lerebours G, Nitenberg A. Angiotensin-converting enzyme inhibition restores flow-dependent and cold pressor test-induced dilations in coronary arteries of hypertensive patients. Circulation 1996 Dec 15; 94(12): 3115–22
Ghiadoni L, Magagna A, Versari D, et al. Different effect of antihypertensive drugs on conduit artery endothelial function. Hypertension 2003 Jun; 41(6): 1281–6
Bots ML, Remme WJ, Grobbee DE, et al. ACE inhibition and endothelial function in the EUROPA Trial: the main findings from the PERFECT substudy [abstract no. 825-8]. J Am Coll Cardiol 2005 Feb 1; 45 (3 Suppl. A): 409
Ferrari R, Remme WJ, EUROPA Investigators, et al. PERTINENT; PERindopril — Thrombosis, InflammatioN, Endothelial dysfunction and Neurohormonal activation Trial [online]. Available from URL: http://www.europa-trial.org/pro/pertinent/pertinent_findings.asp [Accessed 2005 Oct 25]
Asmar R, Topouchian J, Pannier B, et al. Pulse wave velocity as endpoint in large-scale intervention trial: the Complior study. Scientific, Quality Control, Coordination and Investigation Committees of the Complior Study. J Hypertens 2001 Apr; 19(4): 813–8
Asmar RG, London GM, O’Rourke ME, et al. Improvement in blood pressure, arterial stiffness and wave reflections with a very-low-dose perindopril/indapamide combination in hypertensive patient: a comparison with atenolol. Hypertension 2001 Oct; 38(4): 922–6
Girerd X, Giannattasio C, Moulin C, et al. Regression of radial artery wall hypertrophy and improvement of carotid artery compliance after long-term antihypertensive treatment in elderly patients. J Am Coll Cardiol 1998 Apr; 31(5): 1064–73
Kool MJ, Lustermans FA, Breed JG, et al. The influence of perindopril and the diuretic combination amiloride + hydrochlorothiazide on the vessel wall properties of large arteries in hypertensive patients. J Hypertens 1995 Aug; 13(8): 839–48
Guerin AP, Blacher J, Pannier B, et al. Impact of aortic stiffness attenuation on survival of patients in end-stage renal failure. Circulation 2001 Feb 20; 103(7): 987–92
Tropeano A, Boutouyrie P, Pannier B, et al. Pressure-independent and dose-dependent remodelling of the carotid artery after perindopril in diabetic hypertensive patients. J Hypertens 2005; 23 Suppl. 2: S225
Thybo NK, Stephens N, Cooper A, et al. Effect of antihypertensive treatment on small arteries of patients with previously untreated essential hypertension. Hypertension 1995 Apr; 25 (4 Pt 1): 474–81
Buus NH, Bottcher M, Jorgensen CG, et al. Myocardial perfusion during long-term angiotensin-converting enzyme inhibition or beta-blockade in patients with essential hypertension. Hypertension 2004 Oct; 44(4): 465–70
Schwartzkopff B, Brehm M, Mundhenke M, et al. Repair of coronary arterioles after treatment with perindopril in hypertensive heart disease. Hypertension 2000 Aug; 36(2): 220–5
Agabiti-Rosei E, Ambrosioni E, Finardi G, et al. Perindopril versus captopril: efficacy and acceptability in an Italian multicenter trial. Am J Med 1992 Apr 27; 92 Suppl. 4B: 79–83S
Grandi AM, Venco A, Barzizza F, et al. Double-blind comparison of perindopril and captopril in hypertension. Effects on left ventricular morphology and function. Am J Hypertens 1991 Jun; 4(6): 516–20
Grandi AM, Bignotti M, Gaudio G, et al. Ambulatory blood pressure and left ventricular changes during antihypertensive treatment: perindopril versus isradipine. J Cardiovasc Pharmacol 1995 Nov; 26(5): 737–41
Hui Y, Dai Z, Chen X, et al. Effect of perindopril and metoprolol on left ventricular hypertrophy and performance in essential hypertension. Chin Med J 1995 Sep; 108(9): 678–81
Kuperstein R, Sasson Z. Effects of antihypertensive therapy on glucose and insulin metabolism and on left ventricular mass: a randomized, double-blind, controlled study of 21 obese hypertensives. Circulation 2000 Oct 10; 102(15): 1802–6
London GM, Pannier B, Guerin AP, et al. Cardiac hypertrophy, aortic compliance, peripheral resistance, and wave reflection in end-stage renal disease. Comparative effects of ACE inhibition and calcium channel blockade. Circulation 1994 Dec; 90(6): 2786–96
Overlack A, Adamczak M, Bachmann W, et al. ACE-inhibition with perindopril in essential hypertensive patients with concomitant diseases. The Perindopril Therapeutic Safety Collaborative Research Group. Am J Med 1994 Aug; 97(2): 126–34
PERTINENT Investigators. PERTINENT-PERindopril-Thrombosis, Inflammation, Endothelial dysfunction and Neurohormonal activation Trial: a sub-study of the EUROPA study. Cardiovasc Drugs Ther 2003 Jan; 17(1): 83–91
Williams B. Differential impact of the blood pressure lowering drugs on central arterial pressure influences clinical outcomes — principle results of the Conduit Artery Function Evaluation [late breaker abstract no. PS.01 plus oral presentation]. 78th Scientific Sessions of the American Hypertension Association; 2005 Nov 13; Dallas (TX)
Servier Laboratories Ltd. Coversyl®: summary of product characteristics [online]. Available from URL: http://www.emc.medicines.org.uk [Accessed 2005 Nov 4]
Solvay Pharmaceuticals Inc. Aceon: US prescribing and safety information [online]. Available from URL: http://www.solvaypharmaceuticals-us.com [Accessed 2005 Nov 4]
Bellissant E, Giudicelli J-F. Pharmacokinetic-pharmacodynamic model for perindoprilat regional haemodynamic effects in healthy volunteers and in congestive heart failure patients. Br J Clin Pharmacol 2001 Jul; 52(1): 25–33
Fox KM. Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double-blind, placebo-controlled, multicentre trial (the EUROPA study). Lancet 2003 Sep 6; 362(9386): 782–8
Ferrari R. PREAMI: Perindopril and Remodelling in Elderly with Acute Myocardial Infarction. European Society of Cardiology Congress 2005: hotline session: (abstract plus oral presentation), 2005 4 Sep; Stockholm [online]. Available from: URL: http://cic.escardio.org [Accessed 2005 Sep 14]
PROGRESS Collaborative Group. Effects of a perindopril-based blood pressure lowering regimen on cardiac outcomes among patients with cerebrovascular disease. Eur Heart J 2003 Mar; 24(5): 475–84
Dahlof B, Sever PS, Poulter NR, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOTBPLA): a multicentre randomised controlled trial. Lancet 2005 Sep 10; 366(9489): 895–906
Fox KM. Management of coronary artery disease: implications of the EUROPA trial. Br J Cardiol 2004; 11(3): 195–204
EUROPA Investigators. EUROPA; European trial on Reduction Of cardiac evens with Perindopril in stable coronary Artery disease [online]. Available from URL: http://www.europa-trial.org [Accessed 2005 Nov 4]
Daly CA, Fox KM, Remme WJ, et al. The effect of perindopril on cardiovascular morbidity and mortality in patients with diabetes in the EUROPA study: results from the PERSUADE substudy. Eur Heart J 2005 Jul; 26(14): 1369–78
Fox, K. Prevention of events in coronary artery disease with ACE inhibitors: new insights from EUROPA. Oral symposium presented at European Society of Cardiology Congress; 2005 Sept 5; Stockholm
Bertrand ME, Remme WJ, Fox KM, et al. Effects of perindopril on long-term clinical outcome of patients with coronary artery disease and preserved left ventricular function: the EUROPA study [abstract no. P3489]. Annual Congress of the European Society of Cardiology; 2005 Sep 3–7; Stockholm
Deckers JW, Goedhart DM, Bertrand ME, et al. Treatment benefit by perindopril in patients with stable coronary artery disease at different levels of risk [abstract no. 3663]. Annual Congress of the European Society of Cardiology; 2005 Sep 3–7; Stockholm
Cleland JG, Coletta AP, Lammiman M, et al. Clinical trials update from the European Society of Cardiology meeting 2005: CARE-HF extension study, ESSENTIAL, CIBIS-III, SICD, ISSUE-2, STRIDE-2, SOFA, IMAGINE, PREAMI, SIRIUS-II and ACTIVE. Eur J Heart Fail 2005 Oct; 7(6): 1070–5
PREAMI Investigators. PREAMI: Perindopril and Remodelling in Elderly with Acute Myocardial Infarction: study rationale and design. Cardiovasc Drugs Ther 2000 Dec; 14(6): 671–9
PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet 2001 Sep 29; 358(9287): 1033–41
Speirs C, Wagniart F, Poggi L. Perindopril postmarketing surveillance: a 12 month study in 47,351 hypertensive patients. Br J Clin Pharmacol 1998 Jul; 46(1): 63–70
Haiat R, Piot O, Gallois H, et al. Blood pressure response to the first 36 hours of heart failure therapy with perindopril versus captopril. French General Hospitals National College of Cardiologists. J Cardiovasc Pharmacol 1999 Jun; 33(6): 953–9
Anthopoulos L, Apostolou T, Bonoris P, et al. Comparative haemodynamic responses to the first dose of short- and long-acting ACE inhibitors in patients with congestive heart failure. Curr Med Res Opin 2001; 17(4): 290–7
Vitovec J, Spinar J. First-dose hypotension after angiotensin-converting enzyme (ACE) inhibitors in chronic heart failure: a comparison of enalapril and perindopril. Slovak Investigator Group. Eur J Heart Fail 2000 Sep; 2(3): 299–304
Navookarasu NT, Rahman AR, Abdullah I. First-dose response to angiotensin-converting enzyme inhibition in congestive cardiac failure: a Malaysian experience. Int J Clin Pract 1999 Jan-1999 28; 53(1): 25–30
European Medicines Agency: Committee for Medicinal Products for Human Use. Doc. Ref. EMEA/246640/2005 [media release]. 2005
Chobanian AV, Bakris GL, Black HR, et al. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003 Dec; 42(6): 1206–52
Pfeffer MA, Braunwald E, Moye LA, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction: results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med 1992 Sep 3; 327(10): 669–77
Kober L, Torp-Pedersen C, Carlsen JE, et al. A clinical trial of the angiotensin-converting-enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial infarction. Trandolapril Cardiac Evaluation (TRACE) Study Group. N Engl J Med 1995 Dec 21; 333(25): 1670–6
The Acute Infarction Ramipril Efficacy (AIRE) Study Investigators. Effect of ramipril on mortality and morbidity of survivors of acute myocardial infarction with clinical evidence of heart failure. Lancet 1993 Oct 2; 342(8875): 821–8
The CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure: results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med 1987 Jun 4; 316(23): 1429–35
Ambrosioni E, Borghi C, Magnani B. The effect of the angiotensin-converting-enzyme inhibitor zofenopril on mortality and morbidity after anterior myocardial infarction. The Survival of Myocardial Infarction Long-term Evaluation (SMILE) Study Investigators. N Engl J Med 1995 Jan 12; 332(2): 80–5
Pitt B, O’Neill B, Feldman R, et al. The QUinapril Ischemic Event Trial (QUIET): evaluation of chronic ACE inhibitor therapy in patients with ischemic heart disease and preserved left ventricular function. Am J Cardiol 2001 May 1; 87(9): 1058–63
Yusuf S, Sleight P, Pogue J, et al. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med 2000 Jan 20; 342(3): 145–53
Braunwald E, Domanski MJ, Fowler SE, et al. Angiotensin-converting-enzyme inhibition in stable coronary artery disease. N Engl J Med 2004 Nov 11; 351(20): 2058–68
White HD. Should all patients with coronary disease receive angiotensin-converting-enzyme inhibitors? Lancet 2003 Sep 6; 362(9386): 755–7
MacMahon S, Peto R, Cutler J, et al. Blood pressure, stroke, and coronary heart disease. Part 1. Prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet 1990 Mar 31; 335(8692): 765–74
Gomma AH, Fox KM, EUROPA Investigators. The EUROPA Trial: design, baseline demography and status of the substudies. Cardiovasc Drugs Ther 2001 Mar; 15: 169–79
Bonarjee VV, Carstensen S, Caidahl K, et al. Attenuation of left ventricular dilatation after acute myocardial infarction by early initiation of enalapril therapy. CONSENSUS II Multi-Echo Study Group. Am J Cardiol 1993 Nov 1; 72(14): 1004–9
St John Sutton M, Pfeffer MA, Plappert T, et al. Quantitative two-dimensional echocardiographic measurements are major predictors of adverse cardiovascular events after acute myocardial infarction. The protective effects of captopril. Circulation 1994 Jan; 89(1): 68–75
Greenberg B, Quinones MA, Koilpillai C, et al. Effects of long-term enalapril therapy on cardiac structure and function in patients with left ventricular dysfunction: results of the SOLVD echocardiography substudy. Circulation 1995 May 15; 91(10): 2573–81
St John Sutton M, Pfeffer MA, Moye L, et al. Cardiovascular death and left ventricular remodeling two years after myocardial infarction: baseline predictors and impact of long-term use of captopril: information from the Survival and Ventricular Enlargement (SAVE) trial. Circulation 1997 Nov 18; 96(10): 3294–9
Nicolosi GL, Latini R, Marino P, et al. The prognostic value of predischarge quantitative two-dimensional echocardiographic measurements and the effects of early lisinopril treatment on left ventricular structure and function after acute myocardial infarction in the GISSI-3 Trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico. Eur Heart J 1996 Nov; 17(11): 1646–56
Gibbons RJ, Abrams J, Chatterjee K, et al. ACC/AHA 2002 guidelines update for the management of patients with chronic stable angina; a report of the American College of Cardiology/ American Heart Association task force on practice guidelines [online]. Available from URL: http://www.acc.org/clinical/guidelines/stable/stable_clean.pdf [Accessed 2005 Nov 14]
Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction. Executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction). Circulation 2004 Aug 3; 110(5): 588–636
National Institutes for Clinical Excellence. Hypertension: management of hypertension in adults in primary care — clinical guideline 18 [online]. Available from URL: http://www.nice.org.uk/CGO18NICEguideline [Accessed 2005 Nov 24]
Guidelines Committee. 2003 European Society of Hypertension-European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens 2003 Jun; 21(6): 1011–53
Williams B, Poulter NR, Brown MJ, et al. British Hypertension Society guidelines for hypertension management 2004 (BHS-IV): summary. BMJ 2004 Mar 13; 328(7440): 634–40
Pepine CJ, Handberg EM, Cooper-DeHoff RM, et al. A calcium antagonist vs a non-calcium antagonist hypertension treatment strategy for patients with coronary artery disease. The International Verapamil-Trandolapril Study (INVEST): a randomized controlled trial. JAMA 2003 Dec 3; 290(21): 2805–16
Julius S, Kjeldsen SE, Weber M, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet 2004 Jun 19; 363(9426): 2022–31
Turnbull F. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet 2003 Nov 8; 362(9395): 1527–35
Lewington S, Clarke R, Qizilbash N, et al. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002 Dec 14; 360(9349): 1903–13
Poulter NR, Wedel H, Dahlof B, et al. Role of blood pressure and other variables in the differential cardiovascular event rates noted in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA). Lancet 2005 Sep 10; 366(9489): 907–13
Verdecchia P, Reboldi G, Angeli F, et al. Angiotensin-converting enzyme inhibitors and calcium channel blockers for coronary heart disease and stroke prevention. Hypertension 2005 Aug; 46(2): 386–92
Turnbull F. Blood pressure-independent effects for agents inhibiting the renin-angiotensin system. Plenary session. Fifteenth European Meeting on Hypertension; 2005 Jun 17–21; Milan
Strauss MH, Lonn EM, Verma S. Is the jury out? Class specific differences on coronary outcomes with ACE-inhibitors and ARBs: insight from meta-analysis and The Blood Pressure Lowering Treatment Trialists’ Collaboration. Eur Heart J 2005 Nov; 26(22): 2351–3
Novartis. Diovan® (valsartan tablets): US prescribing information. 2005 Aug
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: R. Asmar, L’Institut CardioVasculaire, Paris, France; A.M. Grandi, Department of Clinical Medicine, University of Insubria, Varese, Italy; U. Kaul, Fortis Hospitals, New Delhi, India; H. Purcell, Royal Brompton Hospital and Harefield NHS Trust, London, England; F. Ribichini, University of Piemonte Orientales, Novara, Italy; M.L. Simoons, Erasmus Medical Center — Thoraxcenter, Rotterdam, The Netherlands.
Data Selection
Sources: Medical literature published in any language since 1980 on ‘perindopril’, identified using MEDLINE and EMBASE, supplemented by AdisBase (a proprietary database of Adis International). Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: MEDLINE and AdisBase search terms were ‘perindopril’ and (‘coronary artery disease’ or ‘coronary disease’). EMBASE search terms were ‘perindopril’ and (‘coronary artery disease’ or ‘ischemic heart disease’). Searches were last updated 13 January 2006.
Selection: Studies in patients with coronary artery disease who received perindopril. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Perindopril, coronary artery disease, ACE inhibitors, cardiovascular events, pharmacodynamics, pharmacokinetics, therapeutic use, tolerability
Rights and permissions
About this article
Cite this article
Curran, M.P., McCormack, P.L. & Simpson, D. Perindopril. Drugs 66, 235–255 (2006). https://doi.org/10.2165/00003495-200666020-00010
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200666020-00010