Abstract
▴ Tramadol is a synthetic, centrally acting analgesic. A sustained-release (SR) capsule formulation of tramadol gradually releases active drug, allowing for twice-daily dosing.
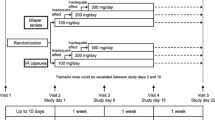
▴ Compared with tramadol SR tablets, tramadol SR capsules produced a smoother plasma concentration profile, with more gradual absorption and lower peak concentrations. There was less intra- and inter-subject variability in tramadol plasma concentrations with SR capsules versus SR tablets.
▴ Tramadol SR capsules had identical bioavailability to tramadol immediate-release (IR) capsules with lower peak concentrations and less fluctuation in plasma concentrations.
▴ Tramadol SR 100mg capsules administered twice daily had equivalent efficacy to tramadol IR 50mg capsules administered four times daily in the treatment of moderate to severe chronic low back pain in a well designed study. Patients receiving tramadol SR capsules were significantly less likely than those receiving tramadol IR capsules to report nausea.
▴ Starting treatment with tramadol SR capsules at a dosage of 50mg twice daily with subsequent dose escalation resulted in improved tolerability in patients with moderate to severe chronic pain.
▴ The lowest tramadol SR capsule dosage of 50mg twice daily (administered to 35% of patients with moderate to severe non-oncological pain) significantly improved pain intensity and frequency in 83.4% and 70.4% of patients, respectively, in a postmarketing observational study evaluating tramadol SR capsules 50–200mg twice daily (n = 3888).


Similar content being viewed by others
Notes
Trade names include Tradonal® Retard, Zamadol® SR caps, Zamudol® LP, Tradonal® SR, Tramadol HCl Meda and Travex®. The use of trade names is for product identification purposes only and does not imply endorsement.
References
Scott LJ, Perry CM. Tramadol: a review of its use in perioperative pain. Drugs 2000 Jul; 60(1): 139–76
Grond S, Sablotzki A. Clinical pharmacology of tramadol. Clin Pharmacokinet 2004; 43(13): 879–923
Cnota PJ, Nowak H, Tagarro I, et al. Tramadol SR formulations: pharmacokinetic comparison of a multiple-units dose (capsule) versus a single-unit dose (tablet). Clin Drug Invest 2005; 25(7): 435–43
Raber M, Schulz H-U, Schürer M, et al. Pharmacokinetic properties of tramadol sustained release capsules. 2nd communication: investigation of relative bioavailability and food interaction. Arzneimittelforschung Drug Res 1999 Jul; 49: 588–93
Raber M, Schulz H-U, Schürer M, et al. Pharmacokinetic properties of tramadol sustained release capsules. 3rd communication: investigation of relative bioavailability under steady state conditions. Arzneimittelforschung Drug Res 1999 Jul; 49: 594–8
Schulz H-U, Raber M, Schürer M, et al. Pharmacokinetic properties of tramadol sustained release capsules: 1st communication: investigation of dose linearity. Arzneimittelforschung Drug Res 1999 Jul; 49: 582–7
Meda. Zamadol SR capsules 50mg, 100mg, 150mg, 200mg: prescribing information [online]. Available from URL: http:// emc.medicines.org.uk [Accessed 2005 Aug 4]
Raber M, Hofmann S, Junge K, et al. Analgesic efficacy and tolerability of tramadol 100mg sustained-release capsules in patients with moderate to severe chronic low back pain. Clin Drug Invest 1999 Jun; 17(6): 415–23
Tagarro I, Herrera J, Barutell C, et al. Effect of a simple dose-escalation schedule on tramadol tolerability: assessment in the clinical setting. Clin Drug Invest 2005; 25(1): 23–31
Vandeputte O, Martens M, Van Boxem K, et al. Assessment in daily practice of the efficacy and the tolerability of tramadol sustained-release (SR) capsules in the treatment of moderate to severe chronic pain of non-oncological origin [abstract no. 1687-P235]. 10th World Congress on Pain; 2002 Aug 17–22; San Diego (CA)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Keating, G.M. Tramadol Sustained-Release Capsules. Drugs 66, 223–230 (2006). https://doi.org/10.2165/00003495-200666020-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200666020-00006