Summary
Abstract
Cefditoren pivoxil (Spectracef®, Meiact®) is a third-generation oral cephalosporin with a broad spectrum of activity against pathogens, including both Gram-positive and -negative bacteria, and is stable to hydrolysis by many common β-lactamases. Cefditoren pivoxil is approved for use in the treatment of acute exacerbations of chronic bronchitis (AECB), mild-to-moderate community-acquired pneumonia (CAP), acute maxillary sinusitis, acute pharyngitis/tonsillitis and uncomplicated skin and skin structure infections (indications may differ between countries).
In clinical trials in adults and adolescents, cefditoren pivoxil demonstrated good clinical and bacteriological efficacy in AECB, CAP, acute maxillary sinusitis, acute pharyngitis/tonsillitis and uncomplicated skin and skin structure infections and was generally well tolerated. Thus, cefditoren pivoxil is a good option for the treatment of adult and adolescent patients with specific respiratory tract or skin infections, particularly if there is concern about Streptococcus pneumoniae with decreased susceptibility to penicillin, or β-lactamase-mediated resistance among the common community-acquired pathogens.
Antibacterial Activity
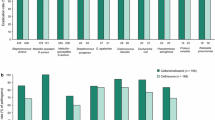
Cefditoren has a broad spectrum of antibacterial activity against a number of the common Gram-positive and -negative pathogens. In vitro studies conducted since 1998 have shown that cefditoren has good activity against meticillin-susceptible Staphylococcus aureus, Streptococcus pyogenes and penicillin-susceptible and -intermediate S. pneumoniae. Minimum inhibitory concentrations of cefditoren required to inhibit 90% of bacterial strains (MIC90) of penicillin-resistant S. pneumoniae were considerably lower than those of cefuroxime and cefdinir. In the ARISE (Antibiotic Resistance Isolates in Southern Europe) project, the MIC90 of cefditoren (0.5 mg/L) against 877 clinical isolates of S. pneumoniae, 16.5% of which were resistant to penicillin, was lower than those of all other antibacterials tested, including cefpodoxime, cefotaxime, amoxicillin/ clavulanic acid and levofloxacin.
Cefditoren was highly active against the Gram-negative organisms Haemophilus influenzae, H. parainfluenzae and Moraxella catarrhalis, including β-lacta-mase-producing strains. Indeed, cefditoren is stable to hydrolysis by many of the common plasmid-mediated β-lactamases, including TEM-1, ROB-1, SHV-1, SHV-3, SHV-10, OXA-5, OXA-12, PSE-1, PSE-2, PSE-3, PSE-4, SAR-1, HMS-1, CARB-4, LCR-1, TLE-1 and OHIO-1. However, cefditoren is susceptible to hydrolysis by a number of plasmid-mediated extended-spectrum β-lactamases (e.g. TEM-3, TEM-4, TEM-5, TOHO-1, SHV-2, SHV-7, SHV-9, SHV-12 and PER-1).
By binding to penicillin-binding proteins, cefditoren inhibits bacterial cell wall synthesis, leading to cell lysis and death of susceptible bacteria. Cefditoren is bactericidal against S. pneumoniae (including penicillin-resistant strains), S. pyogenes, H. influenzae, M. catarrhalis and S. aureus at concentrations of one to four times the MIC.
Pharmacokinetic Properties
Cefditoren is formulated as a pivoxil ester in order to increase bioavailability. After oral administration, cefditoren pivoxil is hydrolysed by intestinal esterases to form cefditoren (the active metabolite) and pivalate. A single 400mg oral dose administered with food afforded a mean maximum plasma concentration (Cmax) of 3.8–4.6 mg/L after 2.4–3.1 hours. Mean area under the plasma concentration-time curve (AUC) values were 11.4–17.4 mg · h/L. After a 7-day course of twice-daily cefditoren pivoxil 400mg, Cmax and AUC values for cefditoren were similar to those after a single dose. Binding of cefditoren to plasma proteins averages 88%, and the mean volume of distribution of cefditoren at steady state is 9.3L.
Cefditoren has been shown to penetrate into bronchial mucosa, epithelial lining fluid, skin blister fluid and tonsillar tissue and, although data are limited, clinically relevant concentrations against common pathogens are achieved in these tissues for at least 4 hours.
Cefditoren is predominantly eliminated by the kidneys as unchanged drug and has a renal clearance of 4.1–5.6 L/h after multiple doses; its elimination half-life is 1.5 hours. Dosage adjustments may be required in patients with renal dysfunction.
Coadministration of cefditoren pivoxil with histamine H2-receptor antagonists or aluminium/magnesium-containing antacids is not recommended because of the resulting decrease in plasma cefditoren concentrations.
Therapeutic Efficacy
The clinical and bacteriological efficacy of oral cefditoren pivoxil in the treatment of adult and adolescent patients with community-acquired respiratory tract infections or skin and skin structure infections has been established in numerous trials conducted in Europe, the US and South Africa.
The clinical and microbiological efficacy of cefditoren pivoxil 200mg twice daily for 5 days was shown to be equivalent to that of cefuroxime axetil 250mg twice daily for 10 days in the treatment of patients with AECB (Anthonisen I and II). Cefditoren pivoxil 200 or 400mg twice daily for 10 days also demonstrated good clinical and bacteriological efficacy in the treatment of AECB.
Clinical cure rates with twice-daily cefditoren pivoxil 200 or 400mg for 14 days (86.5–88.4%) were similar to those with 14-day regimens of amoxicillin/ clavulanic acid or cefpodoxime proxetil in patients with mild-to-moderate CAP. Bacteriological eradication rates of 77.3–85.7%, 79.8% and 91.7% were seen with cefditoren, amoxicillin/clavulanic acid and cefpodoxime proxetil.
In patients with acute maxillary sinusitis, cefditoren pivoxil 200 or 400mg twice daily for 10 days produced clinical cure rates (63.6–94.9%) similar to standard regimens of amoxicillin/clavulanic acid or cefuroxime axetil. Bacterial eradication rates were reported in one study: 72.3% with twice-daily cefditoren pivoxil 200mg and 86.4% with cefuroxime axetil.
Cefditoren pivoxil 200mg twice daily for 5 or 10 days produced clinical cure rates of >92% in patients with acute pharyngitis/tonsillitis, which were similar to those achieved with standard 10-day courses of penicillin V. Microbiological cure rates with cefditoren pivoxil 200mg twice daily for 10 days were 90.4%, compared with 82.7% with penicillin V 250mg four times daily for 10 days in patients with streptococcal pharyngitis/tonsillitis.
Clinical cure rates with cefditoren pivoxil 200 or 400mg twice daily for 10 days (81.5–85.3%) in patients with skin and skin structure infections were similar to those seen with cefuroxime axetil or cefadroxil; corresponding bacteriological eradication rates (54% of isolated pathogens were S. aureus) were 80.9–87.4%, 88.6% and 76.6%.
Tolerability
Cefditoren pivoxil is generally well tolerated, with most adverse events being of mild-to-moderate severity and self-limiting. Gastrointestinal adverse events (e.g. diarrhoea, nausea and abdominal pain) were the most commonly reported adverse events, although they seldom led to treatment discontinuation. Diarrhoea was the most common adverse event with cefditoren pivoxil (incidence of >10% in most trials) and often occurred with a significantly higher incidence than with comparator drugs.
As with other pivalate-producing agents, cefditoren pivoxil can cause transient decreases in plasma carnitine levels and is, thus, contraindicated in patients with primary carnitine deficiency.










Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Kuti JL, Quintiliani R. Cefditoren pivoxil: a novel broad-spectrum oral cephalosporin. Formulary 2001; 36: 265–8 and 271–275
Purdue Pharmaceutical Products L.P. Package insert: Spectracef® tablets (cefditoren pivoxil) [online]. Available from URL: http://www.pharma.com/PI/Prescription/spectracef.pdf [Accessed 2004 Aug 23]
Tedec-Meiji Farma S.A. Summary of product characteristics: Spectracef® film-coated tablets. Madrid: Tedec-Meiji Farma S.A., 2004
Darkes MJ, Plosker GL. Cefditoren pivoxil. Drugs 2002; 62(2): 319–36; discussion 337-8
Kelly LM, Jacobs MR, Appelbaum PC. Comparison of agar dilution, microdilution, E-test, and disk diffusion methods for testing activity of cefditoren against Streptococcus pneumoniae. J Clin Microbiol 1999; 37(10): 3296–9
Johnson DM, Biedenbach DJ, Beach ML, et al. Antimicrobial activity and in vitro susceptibility test development for cefditoren against Haemophilus influenzae, Moraxella catar-rhalis, and Streptococcus species. Diagn Microbiol Infect Dis 2000; 37(2): 99–105
Karlowsky JA, Jones ME, Draghi DC, et al. In vitro susceptibility of recent clinical isolates of pneumococci to the investigational cephalosporin cefditoren. Diagn Microbiol Infect Dis 2002 Jan; 42(1): 59–64
Soriano F, Granizo JJ, Fenoll A, et al. Antimicrobial resistance among clinical isolates of Streptococcus pneumoniae isolated in four southern European countries (ARISE project) from adult patients: results from the cefditoren surveillance program. J Chemother 2003 Apr; 15(2): 107–12
Jones RN, Biedenbach DJ, Croco MAT, et al. In vitro evaluation of a novel orally administered cephalosporin (cefditoren) tested against 1249 recent clinical isolates of Haemophilus influenzae, Moraxella catarrhalis, and Streptococcus pneumoniae. Diagn Microbiol Infect Dis 1998; 31: 573–8
Fuchs PC, Barry AL, Brown SD. Susceptibility of Streptococcus pneumoniae and Haemophilus influenzae to cefditoren, and provisional interpretive criteria. Diagn Microbiol Infect Dis 2000; 37: 265–9
Dubois J, St-Pierre C. Superior inhibitory and bactericidal activity of cefditoren against respiratory tract pathogens [poster no. 370]. 40th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2000 Sep 17–20; Toronto
Ednie LM, Pankuch GA, Jacobs MR, et al. Activity of cefditoren compared with other agents against quinolone and macrolide susceptible and resistant pneumococci [poster no. 378]. 40th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2000 Sep 17–20; Toronto
Beyer J, Shortridge V, Flamm R, et al. In vitro activity of cefditoren against clinical isolates of S. pneumoniae, H. influenzae, and M. Catarrhalis [poster no. 376]. 40th Inter-science Conference on Antimicrobial Agents and Chemotherapy; 2000 Sep 17–20; Toronto
Peric M, Browne FA, Jacobs MR, et al. Activity of nine oral agents against gram-positive and gram-negative bacteria encountered in community-acquired infections: use of pharmacokinetic/pharmacodynamic breakpoints in the comparative assessment of beta-lactam and macrolide antimicrobial agents. Clin Ther 2003 Jan; 25(1): 169–77
Clark CL, Nagai K, Dewasse BE, et al. Activity of cefditoren against respiratory pathogens. J Antimicrob Chemother 2002 Jul; 50(1): 33–41
Seki H, Kasahara Y, Ohta K, et al. Antimicrobial activities of cefditoren against respiratory pathogens isolated from children in Japan. J Infect Chemother 1999 Mar; 5(1): 16–20
Sahm DF, Draghi D, Blosser RS, et al. Comparative activities of cefditoren and other antimicrobials against recent clinical isolates of non-pneumococcal streptococci collected throughout the United States [poster no. 375]. 40th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2000 Sep 17–20; Toronto
Granizo JJ, Fernandez-Roblas R, Coronel P, et al. In vitro susceptibility of 590 isolates of S. pyogenes against cefditoren and 10 other antimicrobials: a multicenter international study in Southern Europe (ARISE Project) [abstract no. P1368]. Clin Microbiol Infect 2002 Apr; 8 Suppl. 1: 320
Soriano F, Granizo JJ, Coronel P, et al. Antimicrobial susceptibility of Haemophilus influenzae, Haemophilus parain-fluenzae and Moraxella catarrhalis isolated from adult patients with respiratory tract infections in four southern European countries: the ARISE project. Int J Antimicrob Agents 2004 Mar; 23(3): 296–9
Nishi J, Yoshinaga M, Tokuda K, et al. Oral antimicrobial susceptibilities of Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis isolates from Japanese children. Int J Antimicrob Agents 2002; 20: 130–5
Sahm DF, Karlowsky JA, Draghi D, et al. Comparative activities of cefditoren and other antimicrobials against recent clinical isolates of H. influenzae and M. catarrhalis collected throughout the United States [poster no. 369]. 40th Inter-science Conference on Antimicrobial Agents and Chemotherapy; 2000 Sep 17–20; Toronto
Karlowsky JA, Critchley IA, Draghi DC, et al. Activity of cefditoren against β-lactamase-positive and -negative Haemophilus influenzae and Moraxella catarrhalis. Diagn Microbiol Infect Dis 2002 Jan; 42(1): 53–8
Balbisi EA. Cefditoren, a new aminothiazolyl cephalosporin. Pharmacotherapy 2002 Oct; 22(10): 1278–93
Medeiros AA. Comparison of oral cephalosporins and their activity against β-lactamase-producing bacteria. Infect Dis Clin Pract 1998 Nov; 7 Suppl. 4: S273–9
Jones RN, Pfaller MA, Jacobs MR, et al. Cefditoren in vitro activity and spectrum: a review of international studies using reference methods. Diagn Microbiol Infect Dis 2001; 41(1–2): 1–14
Spangler SK, Jacobs MR, Appelbaum PC. Time-kill studies on susceptibility of nine penicillin-susceptible and -resistant pneumococci to cefditoren compared with nine other β-lactams. J Antimicrob Chemother 1997; 39: 141–8
Dubois J, St-Pierre C. In vitro study of the post-antibiotic effect and the bactericidal activity of cefditoren and ten other oral antimicrobial agents against upper and lower respiratory tract pathogens. Diagn Microbiol Infect Dis 2000; 37: 187–93
Felmingham D, Robbins MJ, Ghosh G, et al. An in vitro characterization of cefditoren, a new oral cephalosporin. Drugs Exp Clin Res 1994; 20: 127–47
Draghi D, Selman LJ, Karlowsky JA, et al. Bactericidal activity of cefditoren and comparator agents against penicillin-susceptible,-intermediate, and -resistant isolates of Streptococcus pneumoniae [poster no. 373]. 40th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2000 Sep 17–20; Toronto
Soriano F, Coronel P, Gimeno M, et al. Inoculum effect and bactericidal activity of cefditoren and other antibiotics against Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis. Eur J Clin Microbiol Infect Dis 1996; 15: 761–3
Livermore DM. β-lactamase-mediated resistance and opportunities for its control. J Antimicrob Chemother 1998; 41 Suppl. D: 25–41
Ehrhardt AF, Moland ES, Sanders CC. Impact of specific β-lactamase and β-lactamase/porin combinations on the in vitro activity of cefditoren in a genetically defined panel [poster no. 374]. 40th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2000 Sep 17–20; Toronto
Hasegawa K, Yamamoto K, Chiba N, et al. Diversity of ampicillin-resistance genes in Haemophilus influenzae in Japan and the United States. Microb Drug Resist 2003 Spring; 9(1): 39–46
Baquero F. Gram-positive resistance: challenge for the development of new antibiotics. J Antimicrob Chemother 1997; 39 Suppl. A: 1–6
Dessen A, Di Guilmi AM, Vernet T, et al. Molecular mechanisms of antibiotic resistance in gram-positive pathogens. Curr Drug Targets Infect Disord 2001; 1(1): 63–77
Nagai K, Davies TA, Jacobs MR, et al. Effects of amino acid alterations in penicillin-binding proteins (PBPs) 1a, 2b, and 2x on PBP affinities of penicillin, ampicillin, amoxicillin, cefditoren, cefuroxime, cefprozil, and cefaclor in 18 clinical isolates of penicillin-susceptible, -intermediate, and -resistant pneumococci. Antimicrob Agents Chemother 2002 May; 46(5): 1273–80
Craig WA. Interrelationship between pharmacokinetics and pharmacodynamics in determining dosage regimens for broad-spectrum cephalosporins. Diagn Microbiol Infect Dis 1995; 22: 89–96
Craig WA, Andes DR. In vivo pharmacodynamic activity of cefditoren (CDTR) against Streptococcus pneumoniae [abstract no. 2248]. 41st Interscience Conference on Antimicrobial Agents and Chemotherapy; 2001 Dec 16–19; Chicago
Tawara S, Hatano K, Wakai Y, et al. In vivo antibacterial activity of FK041, a new orally active cephalosporin. J Antibiot (Tokyo) 1999; 52(7): 660–5
Sakagami K, Atsumi K, Tamura A, et al. Synthesis and oral activity of ME1207, a new orally active cephalosporin [letter]. J Antibiot (Tokyo) 1990; 43(8): 1047–50
Li JT, Hou F, Lu H, et al. Phase I clinical trial of cefditoren pivoxil (ME 1207): pharmacokinetics in healthy volunteers. Drugs Exp Clin Res 1997; 23: 145–50
Mulford D, Mayer M, Witt G. Effect of age and gender on the pharmacokinetics of cefditoren [poster no. 310]. 40th Inter-science Conference on Antimicrobial Agents and Chemotherapy; 2000 Sep 17–20; Toronto
Mulford D, Mayer M, Witt G. Effect of renal impairment on the pharmacokinetics of cefditoren [poster no. 311]. 40th Inter-science Conference on Antimicrobial Agents and Chemotherapy; 2000 Sep 17–20; Toronto
Mayer M, Mulford D, Witt G. Effect of hepatic impairment on the pharmacokinetics of cefditoren [poster no. 312]. 40th Inter-science Conference on Antimicrobial Agents and Chemotherapy; 2000 Sep 17–20; Toronto
Mayer M, Mulford D, Witt G. Pharmacokinetics of cefditoren in blister fluid and plasma [abstract no. 656]. 40th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2000 Sep 17–20; Toronto, 20
Mayer M, Mulford D, Witt G. Effect of an H2 receptor antagonist or an antacid on the pharmacokinetics of cefditoren [poster no. 313]. 40th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2000 Sep 17–20; Toronto
Mulford D, Mayer M, Witt G. Effect of cefditoren on the pharmacokinetics of ethinyl estradiol [abstract no. 314 plus poster]. 40th Interscience Conference on Antimicrobials Agents and Chemotherapy; 2000 Sep 17–20; Toronto, 13
Kinzig-Schippers M, Hinder M, Göhler K, et al. Tissue penetration of cefditoren (CEE) into bronchial mucosa (BM) and epithelial lining fluid (ELF) in patients undergoing fiberoptic bronchoscopy [abstract no. 946]. 41st Interscience Conference on Antimicrobials Agents and Chemotherapy; 2001 Dec 16–19; Chicago, 24
Guay DR. Review of cefditoren, an advanced-generation, broad-spectrum oral cephalosporin. Clin Ther 2001 Dec; 23(12): 1924–37
Henry DC, Poling TL, Bettis RB, et al. A double-blind, randomized study of cefditoren vs cefuroxime for AECB. J Respir Dis 2001; 22 (8 Suppl.): S69–74
Tucker R, Rhudy J, Hunt B, et al. Safety and efficacy of cefditoren in acute exacerbation of chronic bronchitis (AECB) [abstract no. 836 plus poster]. 40th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2000 Sep 17–20; Toronto, 495
Tedec-Meiji Farma S.A. Efficacy of cefditoren-pivoxil compared to cefuroxime-axetil in patients with acute exacerbation of chronic bronchitis in adults. Tedec-Meiji Farma S.A., 2001 (Data on file)
Fogarty CM, Cyganowski M, Palo WA, et al. A comparison of cefditoren pivoxil and amoxicillin/clavulanate in the treatment of community-acquired pneumonia: a multicenter, prospective, randomized, investigator-blinded, parallel-group study. Clin Ther 2002 Nov; 24(11): 1854–70
van Zyl L, le Roux JG, LaFata JA, et al. Cefditoren pivoxil versus cefpodoxime proxetil for community-acquired pneumonia: results of a multicenter, prospective, randomized, double-blind study. Clin Ther 2002 Nov; 24(11): 1840–53
Comparative safety and efficacy of cefditoren pivoxil and Augmentin® (amoxicillin/clavulanate potassium) in the treatment of patients with acute maxillary sinusitis. TAP Holdings Inc., 1999 (Data on file)
Multicentre, prospective, comparative, phase III study of cefditoren-pivoxil (CDTR-PI) versus cefuroxime-axetil in the treatment of acute community-acquired sinusitis (ACAS) in adults. Tedec-Meiji Farma S.A., 2001 (Data on file)
Gooch W, Marsh D, Stickler T, et al. Cefditoren is safe and effective treatment for streptococcal pharyngitis [abstract no. 837 plus poster]. 40th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2000 Sep 17–20; Toronto, 495
Multicentre phase III, randomised, comparative study: cefditoren pivoxil (ME1207) with penicillin V in the treatment of acute bacterial pharyngotonsillitis in adults. Tedec-Meiji Farma S.A., 2001 (Data on file)
Bucko AD, Hunt BJ, Kidd SL, et al. Randomized, double-blind, multicenter comparison of oral cefditoren 200 or 400 mg bid with either cefuroxime 250 mg bid or cefadroxil 500 mg bid for the treatment of uncomplicated skin and skin-structure infections. Clin Ther 2002; 24(7): 1134–47
Chow J, Russell M, Volk S, et al. Efficacy of cefditoren pivoxil (CDTR) vs amoxicillin/clavulanate (AMX/CLV) in acute maxillary sinusitis (AMS) [abstract no. 835]. 40th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2000 Sep 17–20; Toronto, 495
Brass EP. Pivalate-generating prodrugs and carnitine homeostasis in man. Pharmacol Rev 2002; 54(4): 589–98
Brass EP, Mayer MD, Mulford DJ, et al. Impact on carnitine homeostasis of short-term treatment with the pivalate prodrug cefditoren pivoxil. Clin Pharmacol Ther 2003 Apr; 73(4): 338–47
Donowitz GR, Mandell GL. Cephalosporins. In: Mandell GL, Douglas RG, Bennett JE, editors. Principles and practice of infectious diseases. 3rd ed. New York: Churchill Livingstone, 1990: 246–57
Perry CM, Scott LJ. Cefdinir: a review of its use in the management of mild-to-moderate bacterial infections. Drugs 2004; 64(13): 1433–64
Heffelfinger JD, Dowell SF, Jorgensen JH, et al. Management of community-acquired pneumonia in the era of pneumococcal resistance: a report from the Drug-Resistant Streptococcus pneumoniae Therapeutic Working Group. Arch Intern Med 2000 May 22; 160(10): 1399–408
Jacobs MR, Felmingham D, Appelbaum PC, et al. The Alexander Project 1998–2000: susceptibility of pathogens isolated from community-acquired respiratory tract infection to commonly used antimicrobial agents. J Antimicrob Chemother 2003 Aug; 52(2): 229–46
Niederman MS, Mandell LA, Anzueto A, et al. Guidelines for the management of adults with community-acquired pneumonia: diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med 2001 Jun; 163(7): 1730–54
Anon JB, Jacobs MR, Poole MD, et al. Antimicrobial treatment guidelines for acute bacterial rhinosinusitis. Otolaryngol Head Neck Surg 2004 Jan; 130 (1 Suppl.): 1–45
Baiter MS, La Forge J, Low DE, et al. Canadian guidelines for the management of acute exacerbations of chronic bronchitis: executive summary. Can Respir J 2003; 10(5): 248–58
British Thoracic Society Standards of Care Committee. BTS guidelines for the management of community acquired pneumonia in adults. Thorax 2001 Dec; 56 Suppl. IV: 1–64
Bisno AL, Gerber MA, Gwaltney JM Jr, et al. Practice guidelines for the diagnosis and management of group A streptococcal pharyngitis: Infectious Diseases Society of America. Clin Infect Dis 2002 Jul 15; 35(2): 113–25
Mandell LA, Bartlett JG, Dowell SF, et al. Update of practice guidelines for the management of community-acquired pneumonia in immunocompetent adults. Clin Infect Dis 2003 Dec 1; 37(11): 1405–33
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: E.M. Ambizas, College of Pharmacy and Allied Health Professions, St John’s University, New York, New York, USA; E.A. Balbisi, College of Pharmacy and Allied Health Professions, St John’s University, New York, New York, USA; I.M. Hoepelman, Department of Medicine, University Hospital Utrecht, Utrecht, The Netherlands; J.L. Kuti, Center for Anti-Infective Research and Development, Hartford Hospital, Hartford, Connecticut, USA; J. Nishi, Department of Pediatrics, Kagoshima University, Sakuragaoka, Japan; C.E. Nord, Huddinge University Hospital, Karolinska Institute, Stockholm, Sweden; S.R. Norrby, Swedish Institute for Infectious Disease Control, Solna, Sweden; F. Scaglione, Department of Pharmacology, University of Milan, Milan, Italy.
Data Selection
Sources: Medical literature published in any language since 1980 on cefditoren pivoxil, identified using Medline and EMBASE, supplemented by AdisBase (a proprietary database of Adis International). Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: Medline search terms were ‘cefditoren pivoxil’ or ‘ME-1207’. EMBASE search terms were ‘cefditoren pivoxil’ or ‘ME-1207’. AdisBase search terms were ‘cefditoren-pivoxil’ or ‘ME1207’. Searches were last updated 14 October 2004.
Selection: Studies in patients with bacterial infections who received cefditoren pivoxil. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Cefditoren pivoxil, bacterial infections, cephalosporin, pharmacodynamics, pharmacokinetics, therapeutic use, tolerability.
Rights and permissions
About this article
Cite this article
Wellington, K., Curran, M.P. Cefditoren Pivoxil. Drugs 64, 2597–2618 (2004). https://doi.org/10.2165/00003495-200464220-00009
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200464220-00009