Summary
Abstract
Cefdinir (Omnicef®) is an oral third-generation cephalosporin with good in vitro activity against many pathogens commonly causative in community-acquired infections. The drug provides good coverage against Haemophilus influenzae, Moraxella catarrhalis and penicillin-susceptible Streptococcus pneumoniae, the most common respiratory tract pathogens. Cefdinir is stable to hydrolysis by commonly occurring plasmid-mediated β-lactamases and retains good activity against β-lactamase-producing strains of H. influenzae and M. catarrhalis. The drug distributes into various tissues (e.g. sinus and tonsil) and fluids (e.g. middle ear), and has a pharmacokinetic profile that allows for once-or twice-daily administration.
Cefdinir, administered for 5 or 10 days, has shown good clinical and bacteriological efficacy in the treatment of a wide range of mild-to-moderate infections of the respiratory tract and skin in adults, adolescents and paediatric patients in randomised, controlled trials.
In adults and adolescents, cefdinir is an effective treatment for both lower (acute bacterial exacerbations of chronic bronchitis [ABECB], communityacquired pneumonia) and upper (acute bacterial rhinosinusitis, streptococcal pharyngitis) respiratory tract infections, and uncomplicated skin infections. Its bacteriological and clinical efficacy in patients with lower respiratory tract infections was equivalent to that of comparator agents (cefprozil [bacteriological only], loracarbef, cefuroxime axetil and cefaclor). In one trial in patients with ABECB, cefdinir produced a higher rate of clinical cure than cefprozil (95% CIs indicated nonequivalence). Cefdinir also produced good clinical and bacteriological responses equivalent to responses with amoxicillin/clavulanic acid in patients with acute bacterial rhinosinusitis. In addition, it was at least as effective as penicillin V (phenoxymethylpenicillin) in streptococcal pharyngitis/tonsillitis and as effective as cefalexin in uncomplicated skin infections.
In paediatric patients aged ≥6 months, cefdinir showed similar efficacy to that of amoxicillin/clavulanic acid or cefprozil in acute otitis media, and cefalexin in uncomplicated skin infections. Cefdinir given for 5 or 10 days was at least as effective as penicillin V for 10 days in patients with streptococcal pharyngitis/ tonsillitis.
Cefdinir is usually well tolerated. Diarrhoea was the most common adverse event in trials in all age groups. Although the incidence of diarrhoea in cefdinir recipients was generally higher than in adults and adolescents treated with comparators, discontinuation rates due to adverse events were generally similar for cefdinir and comparator groups.
In conclusion, cefdinir is a third-generation cephalosporin with a broad spectrum of antibacterial activity encompassing pathogens that are commonly causative in infections of the respiratory tract or skin and skin structure. Depending on the infection being treated, cefdinir can be administered as a convenient once-or twice-daily 5-or 10-day regimen. Clinical evidence indicates that cefdinir is an effective and generally well tolerated drug with superior taste over comparator antibacterial agents and is therefore a good option for the treatment of adults, adolescents and paediatric patients with specific mild-to-moderate respiratory tract or skin infections, particularly in areas where β-lactamase-mediated resistance among common community-acquired pathogens is a concern.
Antibacterial Activity
Cefdinir has a broad spectrum of antibacterial activity encompassing a number of Gram-positive and -negative pathogens that are commonly causative in community-acquired respiratory and skin infections. Results of many studies published since 1995 show that cefdinir has good activity against penicillin-susceptible strains of Streptococcus pneumoniae, S. pyogenes and methicillin-susceptible Staphylococcus aureus, including β-lactamase-producers. However, in common with many other β-lactam antibacterials, cefdinir shows less activity against penicillin-intermediate and -resistant pneumococci and is inactive against methicillin-resistant S. aureus.
Cefdinir is also active against the important respiratory tract Gram-negative pathogens Haemophilus influenzae and Moraxella catarrhalis, including β-lactamase-producing strains of both micro-organisms. In addition, the drug shows good in vitro activity against H. parainfluenzae. Cefdinir is stable to hydrolysis by many plasmid-encoded β-lactamases, including TEM-1, TEM-2, TEM-6, TEM-7, TEM-9, TEM-10, HMS-1, CAZ-2, SHV-1, OXA-1, OXA-2, OXA-3 and P99 type 1a. However, it is susceptible to hydrolysis by TEM-3, TEM-4, TEM-5, PSE-2, P99 type 1c, SHV2, SHV-3, SHV-4, SHV-5, MEN-1, K-l, CARB-1, CARB-2, CARB-3 and OXA-4 β-lactamases.
The bactericidal effect of cefdinir is achieved by its binding to penicillinbinding proteins. This leads to damage of the cell wall, cell lysis and death of susceptible bacteria. Cefdinir is rapidly bactericidal against a number of pathogens at minimum bactericidal concentrations of two to four times the minimum inhibitory concentration. Cefdinir has also shown efficacy in several animal models of infection, including pneumonia caused by H. influenzae or penicillinsusceptible strains of S. pneumoniae and subcutaneous abscesses induced by S. aureus.
Pharmacokinetic Properties
In adults, mean maximum plasma cefdinir concentrations (Cmax) were 1.6 and 2.87 mg/L after single doses of 300mg and 600mg at a mean time of ≈3 hours. In paediatric patients, mean cefdinir Cmax values were 2.3 and 3.86 mg/L approximately 2 hours after administration of 7 and 14 mg/kg doses of the suspension. Cefdinir has a linear pharmacokinetic profile over the 200–400mg dose range, but displays nonlinear pharmacokinetics at a higher dose (600mg). At therapeutic dosages, cefdinir does not accumulate in the plasma of individuals with normal renal function.
Cefdinir has an estimated bioavailability of 21% and 16% after administration of single 300 and 600mg capsules, and an estimated absolute bioavailability of 25% after administration of the suspension. The rate and extent of absorption of cefdinir decrease, although not clinically significantly, when the drug is taken with a high-fat meal. Thus, cefdinir may be taken without regard to food.
The mean volume of distribution of cefdnir is 1.56–2.09 L/kg in adults and 0.67 L/kg in paediatric patients. The drug is 60–73% plasma protein bound. Cefdinir is widely distributed and achieves clinically relevant concentrations in bronchial mucosa, epithelial lining fluid, tonsillar tissue, sinus tissue, skin blister fluid and middle ear fluid.
Cefdinir is not metabolised to an appreciable extent and is eliminated via the kidneys. After single oral doses of 300 or 600mg, renal clearance was ≈2 mL/min/ kg and apparent oral clearance values were 11.6 and 15.5 mL/min/kg. The plasma elimination half-life of cefdinir is 1.5–1.7 hours in adults and 1.2–1.5 hours in healthy infants and children.
The pharmacokinetics of cefdinir are altered in patients with renal impairment and dosage modification is required for those with a creatinine clearance (CLcr) of <30 mL/min (<1.8 L/h).
When coadministered with aluminium/magnesium-containing antacids or iron supplements, the gastrointestinal absorption of cefdinir is impaired. Thus, the manufacturer recommends that cefdinir is taken ≥2 hours before or after these agents. Like other β-lactam antibacterials, cefdinir interacts with probenecid and marked increases in systemic exposure to cefdinir occur when the two drugs are coadministered. Reddish stools, resulting from the formation of a nonabsorbable complex between cefdinir (or its breakdown products) and iron, have been reported in some patients.
Therapeutic Efficacy
Oral cefdinir 300mg twice daily or 600mg once daily in adults and adolescents, or 14 mg/kg/day in one or two divided daily doses in paediatric patients, administered for 5 or 10 days, has shown good clinical and bacteriological efficacy at least equivalent to that of oral comparator agents in randomised, controlled trials conducted in patients with a wide range of infections. In most trials, patients had documented pathogens at baseline.
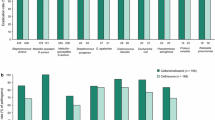
Adults and Adolescents: At test-of-cure (TOC) visits, cefdinir 300mg twice daily or 600mg once daily demonstrated clinical and bacteriological efficacy equivalent to that of cefprozil (bacteriological only), loracarbef and cefuroxime axetil in adults and adolescents with acute bacterial exacerbations of chronic bronchitis (ABECB). In one trial, cefdinir 300mg twice daily for 5 days produced a higher clinical cure rate (80.3%) than cefprozil 500mg twice daily for 10 days (71.9%): clinical cure rates were not equivalent based on 95% confidence intervais. At long-term follow-up (up to 42 days post-treatment), clinical cure and bacteriological eradication rates remained high (>80% for cefdinir and the comparator groups).
In a single trial in adults and adolescents with community-acquired pneumonia (CAP), cefdinir 300mg twice daily for 10 days was equivalent to cefaclor 500mg three times daily for 10 days in terms of both clinical and bacteriological efficacy at the TOC assessments. Clinical success rates with cefdinir and cefaclor were 89% and 86%, respectively, and bacteriological eradication rates were >90% for both groups.
Cefdinir 300mg twice daily or 600mg once daily for 10 days showed clinical and bacteriological efficacy equivalent to that of standard regimens of amoxicillin/ clavulanic acid in trials conducted in adults and adolescents with acute bacterial rhinosinusitis. Clinical responses (clinical cure, or cure plus improvement) were achieved in ≥87% of patients at the TOC visits.
Adults and adolescents with streptococcal pharyngitis/tonsillitis were also successfully treated with cefdinir 300mg twice daily (for 5 or 10 days) or 600mg once daily for 10 days. Cefdinir was at least as effective as penicillin V (250mg four times daily for 10 days). High rates of eradication of S. pyogenes were reported with cefdinir (88.5–91.7%) and both eradication rates and clinical cure rates with 10-day courses of cefdinir were significantly (p ≤0.02) higher than rates achieved with penicillin V at the TOC assessment. Cefdinir 300mg twice daily for 10 days was also an effective treatment for adults and adolescents with skin and skin structure infections and showed clinical and bacteriological efficacy similar to that of cefalexin.
Paediatric Patients: In the treatment of paediatric patients with acute otitis media, cefdinir, administered as a suspension, at a dosage of 7 mg/kg twice daily for 5 or 10 days or 14 mg/kg once daily for 10 days, showed similar clinical efficacy to that of standard regimens of amoxicillin/clavulanic acid or cefprozil.
Cefdinir was also an effective treatment for paediatric patients with streptococcal pharyngitis/tonsillitis. In two comparative trials, rates of eradication of S. pyogenes in patients treated with cefdinir 14 mg/kg/day for 5 or 10 days were significantly (p < 0.001) higher (>90%) than in recipients of 10-day courses of penicillin V 40 mg/kg/day (≈70%). In addition, clinical cure rates with 10-day courses of cefdinir (≥96%) were significantly (p = 0.001) higher than the clinical cure rate (86.3%) in the penicillin V treatment group. Cefdinir 7 mg/kg twice daily for 10 days showed good clinical and bacteriological efficacy, equivalent to that of cefalexin, in paediatric patients with uncomplicated skin and skin structure infections.
Tolerability
Cefdinir was generally well tolerated by adults, adolescents and paediatric patients in randomised clinical trials that enrolled patients with respiratory tract or skin infections. Most reported adverse events were minor and self-limiting and discontinuation rates due to adverse events were low (1–6%). As with most antibacterials, pseudomembranous colitis has occasionally been reported in recipients of cefdinir. Overgrowth of Clostridium difficile should therefore be considered in patients presenting with diarrhoea during treatment.
Adults and Adolescents: According to pooled data from US clinical trials, diarrhoea (incidence 15%) was the most common adverse event in adults and adolescents receiving cefdinir capsules at therapeutic dosages. Similarly, in individual randomised trials, diarrhoea (usually mild) was the most common event (incidence 7.8–33%) in patients treated with cefdinir 300mg twice daily or 600mg once daily. Nausea and headache were the other most frequently reported events (incidence ≤3%) in the pooled analysis.
The incidence of most adverse events (e.g. nausea and headache) with cefdinir is similar to that reported for cefaclor, cefprozil, cefuroxime axetil, loracarbef, penicillin V and amoxicillin/clavulanic acid. However, in comparative trials, the incidence of diarrhoea was significantly (all p ≤0.04) higher in recipients of cefdinir than in those treated with cefaclor, cefprozil, cefalexin, loracarbef or penicillin V. The overall incidence of adverse events in cefdinir treatment groups was also significantly (p ≤0.008) higher than in recipients of cefaclor, loracarbef or cefalexin. However, discontinuation rates due to adverse events were similar for cefdinir and the comparator groups in all but two trials in which discontinuation rates were significantly lower (p ≤0.02) with cefdinir than with amoxicillin/ clavulanic acid.
Paediatric Patients: In a pooled analysis of US clinical trials, and in individual randomised trials, diarrhoea (incidence 8% in the pooled analysis, 2.9–15.9% in the individual trials) was the most common adverse event in paediatric patients receiving cefdinir suspension at therapeutic dosages. Other adverse events included rash and vomiting (incidence ≤3%). In comparative trials, the incidence of adverse events with cefdinir suspension was similar to that reported for cefprozil, penicillin V or cefalexin suspensions. In addition, the incidence of diarrhoea with cefdinir was broadly similar to that reported for cefprozil, penicillin V suspension or amoxicillin/clavulanic acid suspensions. The overall incidence of adverse events with amoxicillin/clavulanic acid was significantly higher than in recipients of cefdinir 14 mg/kg once daily (26.2% vs 16.7%; p = 0.01) in one comparative trial in patients with acute otitis media. In a similar trial, diarrhoea occurred significantly (p < 0.001) more frequently with amoxicillin/clavulanic acid (35%) than with cefdinir 7 mg/kg twice daily (13%) or 14 mg/kg once daily (10%). Rates of treatment discontinuation due to adverse events were similar in recipients of cefdinir, cefprozil or penicillin V. Discontinuation rates in cefdinir recipients were similar to or lower than rates reported for patients treated with amoxicillin/ clavulanic acid.
Dosage and Administration
Cefdinir is approved in the US for the treatment of adults, adolescents and paediatric patients with mild-to-moderate bacterial infections caused by susceptible strains of specific pathogens. The drug is available as capsule and strawberryflavoured suspension formulations and can be administered without regard to food. When given concomitantly, antacids containing magnesium/aluminium, and iron supplements, reduce the the absorption of cefdinir. These agents should be taken ≥2 hours before or after administration of cefdinir.
In adults and adolescents, cefdinir is approved for the treatment of CAP, ABECB, acute maxillary rhinosinusitis, pharyngitis/tonsillitis, and uncomplicated skin and skin structure infections. Approved dosages are 300mg every 12 hours for 10 days (for CAP, uncomplicated skin and skin structure infections, and acute maxillary rhinosinusitis), 300mg every 12 hours for 5 or 10 days (ABECB and pharyngitis/tonsillitis) and 600mg every 24 hours for 10 days (ABECB, acute maxillary rhinosinusitis and pharyngitis/tonsillitis). Dosage modification is required for patients with renal impairment (CLcr <30 mL/min; <1.8 L/h); the dosage should be reduced to 300mg once daily.
In paediatric patients aged 6 months to 12 years, cefdinir is approved for the treatment of acute otitis media, acute maxillary rhinosinusitis, pharyngitis/tonsillitis and uncomplicated skin and skin structure infections: approved dosages are 7 mg/kg every 12 hours for 5–10 days or 14 mg/kg every 24 hours for 10 days (acute otitis media, pharyngitis/tonsillitis), 7 mg/kg every 12 hours or 14 mg/kg every 24 hours for 10 days (acute maxillary rhinosinusitis), and 7 mg/kg every 12 hours for 10 days (skin and skin structure infections). In paediatric patients with renal impairment (CLcr <30 mL/min/1.73m2), the recommended dosage of cefdinir is 7 mg/kg (to a maximum of 300mg) once daily.















Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Guay DRP. Cefdinir: an expanded-spectrum oral cephalosporin. Ann Pharmacother 2000 Dec; 34(12): 1469–77
Omnicef (cefdinir) prescribing information. North Chicago (IL): Abbott Laboratories, 2004
Appelbaum PC. Resistance among Streptococcus pneumoniae: implications for drug selection. Clin Infect Dis 2002 June 15; 34(12): 1613–20
Doern GV, Pfaller MA, Kugler K, et al. Prevalence of antimicrobial resistance among respiratory tract isolates of Streptococcus pneumoniae in North America: 1997 results from the SENTRY Antimicrobial Surveillance Program. Clin Infect Dis 1998 Oct; 27(4): 764–70
Sader H, Fritsche T, Mutnick A, et al. Contemporary evaluation of the in vitro activity and spectrum of cefdinir compared with other orally administered antimicrobials tested against common respiratory tract pathogens (2000–2002). Diag Mic Infect Dis 2003; 47: 515–25
Yamaguchi K, Domon H, Miyazaki S, et al. In vitro and in vivo antibacterial activities of CS-834, a new oral carbapenem. Antimicrob Agents Chemother 1998 Mar; 42(3): 555–63
Leelarasamee A, Trakulsomboon S, Komolpis P. In vitro anti-staphylococcal activity of cefdinir. J Infect Dis Antimicrob Agents 1999 Jan–Feb 30; 16: 17–20
Leelarasamee A, Tian-grim S, Charoensuk B, et al. In vitro activity of cefdinir against community-acquired bacterial pathogens. J Infect Dis Antimicrob Agents 1996 Jan–Feb 30; 13: 1–5
Tamura S, Miyazaki S, Tateda K, et al. In vivo antibacterial activities of sanfetrinem cilexetil, a new oral tricyclic antibiotic. Antimicrob Agents Chemother 1998 Jul; 42(7): 1858–61
Marchese A, Saverino D, Debbia EA, et al. Antistaphylococcal activity of cefdinir, a new oral third-generation cephalosporin, alone and in combination with other antibiotics, at supra-and sub-MIC levels. J Antimicrob Chemother 1995 Jan; 35(1): 53–66
Watanabe Y, Hatano K, Matsumoto Y, et al. In vitro antibacterial activity of FK041, a new orally active cephalosporin. J Antibiot (Tokyo) 1999 Jul; 52(7): 649–59
Sakagawa E, Otsuki M, Oh T, et al. In-vitro and in-vivo antibacterial activities of CS-834, a new oral carbapenem. J Antimicrob Chemother 1998 Oct; 42(4): 427–37
Tsuji M, Ishii Y, Ohno A, et al. In vitro and in vivo antibacterial activities of S-1090, a new oral cephalosporin. Antimicrob Agents Chemother 1995; 39(11): 2544–51
Miyazaki S, Hosoyama T, Furuya N, et al. In vitro and in vivo antibacterial activities of L-084, a novel oral carbapenem, against causative organisms of respiratory tract infections. Antimicrob Agents Chemother 2001 Jan; 45(1): 203–7
Hikida M, Itahashi K, Igarashi A, et al. In vitro antibacterial activity of LJC 11,036, an active metabolite of L-084, a new oral carbapenem antibiotic with potent antipneumococcal activity. Antimicrob Agents Chemother 1999 Aug; 43(8): 2010–6
Paek KS, Kim MY, Lee CS, et al. In vitro and in vivo activities of LB 10827, a new oral cephalosporin, against respiratory pathogens. Antimicrob Agents Chemother 2000 Dec; 44(12): 3272–7
Biedenbach DJ, Jones RN, Pfaller MA. Activity of BMS284756 against 2,681 recent clinical isolates of Haemophilus influenzae and Moraxella catarrhalis: report from the SENTRY Antimicrobial Surveillance Program (2000) in Europe, Canada and the United States. Diagn Microbiol Infect Dis 2001 April; 39(4): 245–50
Jones ME, Blosser-Middleton RS, Critchley IA, et al. Activity of faropenem, a new furanem, against European respiratory pathogens collected during 2000–2001: a comparison with other β-lactam agents [letter]. J Antimicrob Chemother 2003 Jan; 51(1): 196–9
Blandino G, Aleo G, Caccamo F, et al. In vitro activity of cefdinir against respiratory pathogens isolated in Sicily with reference to beta-lactamase production. J Chemother 1996 Jun; 8(3): 193–9
Okamoto H, Miyazaki S, Tateda K, et al. Comparative in vitro activity of telithromycin (HMR 3647), three macrolides, amoxycillin, cefdinir and levofloxacin against gram-positive clinical isolates in Japan. J Antimicrob Chemother 2000 Nov; 46(5): 797–802
Spangler SK, Jacobs MR, Appelbaum PC. Activities of RPR 106972 (a new oral streptogramin), cefditoren (a new oral cephalosporin), two new oxazolidinones (U-100592 and U-100766), and other oral and parenteral agents against 203 penicillin-susceptible and -resistant pneumococci. Antimicrob Agents Chemother 1996 Feb; 40(2): 481–4
Black J, Moland ES, Chartrand SA, et al. Activity of oral agents against pediatric isolates of Streptococcus pneumoniae. Diag Microbiol Infect Dis 2001 Mar; 39(3): 195–7
Clark CL, Nagai K, Dewasse BE, et al. Activity of cefditoren against respiratory pathogens. J Antimicrob Chemother 2002 Jul; 50(1): 33–41
Doern GV, Heilmann KP, Huynh HK, et al. Antimicrobial resistance among clinical isolates of Streptococcus pneumoniae in the United States during 1999–2000, including a comparison of resistance rates since 1994–1995. Antimicrob Agents Chemother 2001 Jun; 45(6): 1721–9
Mason Jr EO, Lamberth LB, Wald ER, et al. In vitro activities of cethromycin (ABT-773), a new ketolide, against Streptococcus pneumoniae strains that are not susceptible to penicillin or macrolides. Antimicrob Agents Chemother 2003 Jan; 47(1): 166–9
Uno Y. Surveillance of susceptibility to antibacterial agents of Streptococcus pneumoniae isolated from middle-ear acute media in infants and children. Jpn J Chemother 2002; 50(12): 854–69
Jacobs MR, Bajaksouzian S, Windau A, et al. Effects of various test media on the activities of 21 antimicrobial agents against Haemophilus influenzae. J Clin Microbiol 2002 Sep; 40(9): 3269–76
Hoban D, Felmingham D. The PROTEKT surveillance study: antimicrobial susceptibility of Haemophilus influenzae and Moraxella catarrhalis from community-acquired respiratory tract infections. J Antimicrob Chemother 2002 Sep; 50 Suppl. S1: 49–59
Peric M, Browne FA, Jacobs MR, et al. Activity of nine oral agents against gram-positive and gram-negative bacteria encountered in community-acquired infections: use of pharmacokinetic/ pharmacodynamic breakpoints in the comparative assessment of beta-lactam and macrolide antimicrobial agents. Clin Ther 2003 Jan; 25(1): 169–77
Milatovic D, Schmitz FJ, Verhoef J, et al. In vitro activity of faropenem against 5460 clinical bacterial isolates from Europe [letter]. J Antimicrob Chemother 2002 Aug; 50(2): 293–9
Seki H, Kasahara Y, Ohta K, et al. Antimicrobial activities of cefditoren against respiratory pathogens isolated from children in Japan. J Infect Chemother 1999 Mar; 5(1): 16–20
Jacobs M, Felmingham D, Appelbaum P, et al. The Alexander Project 1998–2000: susceptibility of pathogens isolated from community-acquired respiratory tract infection to commonly used antimicrobial agents. J Antimicrob Chemother 2003; 52: 229–46
National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: approved standard. 6th ed. NCCLS Document M7-A 6. Wayne (PA): NCCLS, 2003; 20 (2)
National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: approved standard. 4th ed. NCCLS Document M7-A4, V. Villanova (PA): NCCLS, 1997; 17 (1)
Bauernfeind A, Jungwirth R. Antibacterial activity of cefpodoxime in comparison with cefixime, cefdinir, cefetamet, ceftibuten, loracarbef, cefprozil, BAY 3522, cefuroxime, cefaclor and cefadroxil. Infection 1991 Sep–Oct 31; 19(5): 353–62
Briggs BM, Jones RN, Erwin ME, et al. In vitro activity evaluations of cefdinir (FK482, CI-983, and PD134393): a novel orally administered cephalosporin. Diagn Microbiol Infect Dis 1991 Sep–Oct 31; 14(5): 425–34
Appelbaum PC. Cefdinir: a review of its antibacterial and therapeutic potential in community-acquired infections. Clin Drug Invest 1995; 9 Suppl. 3: 54–64
Neu HC, Saha G, Chin NX. Comparative in vitro activity and beta-lactamase stability of FK482, a new oral cephalosporin. Antimicrob Agents Chemother 1989 Oct; 33(10): 1795–800
Wise R, Andrews JM, Thornber D. The in-vitro activity of cefdinir (FK482), a new oral cephalosporin. J Antimicrob Chemother 1991 Aug; 28(2): 239–48
Sultan T, Baltch AL, Smith RP, et al. In vitro activity of cefdinir (FK482) and ten other antibiotics against gram-positive and gram-negative bacteria isolated from adult and pediatric patients. Chemotherapy 1994 Mar–Apr 30; 40(2): 80–91
Linares J, Alonso T, Perez JL, et al. Decreased susceptibility of penicillin-resistant pneumococci to twenty-four beta-lactam antibiotics. J Antimicrob Chemother 1992 Sep; 30(3): 279–88
Spangler SK, Jacobs MR, Appelbaum PC. In vitro susceptibilities of 185 penicillin-susceptible and -resistant pneumococci to WY-49605 (SUN/SY 5555), a new oral penem, compared with those to penicillin G, amoxicillin, amoxicillin-clavulanate, cefixime, cefaclor, cefpodoxime, cefuroxime, and cefdinir. Antimicrob Agents Chemother 1994 Dec; 38(12): 2902–4
Spangler SK, Jacobs MR, Pankuch GA, et al. Susceptibility of 170 penicillin-susceptible and penicillin-resistant pneumococci to six oral cephalosporins, four quinolones, desacetylcefotaxime, Ro 23-9424 and RP 67829. J Antimicrob Chemother 1993 Feb; 31(2): 273–80
Mine Y, Kamimura T, Watanabe Y, et al. In vitro antibacterial activity of FK482, a new orally active cephalosporin. J Antibiot (Tokyo) 1988 Dec; 41(12): 1873–87
Jones RN. In vitro antibacterial activity of oral cephalosporins: a selective and comparative review. Clin Drug Invest 1995; 9 Suppl. 3: 22–30
Jones R, Sader H, Fritsche T, et al. Potency and spectrum reevaluation of cefdinir tested against pathogens causing skin and soft tissue infections: a sample of North American isolates [abstract no. 33.009]. 11th International Congress on Infectious Diseases; 2004 Mar 4–7; Cancun
Sader H, Biedenbach D, Streit J, et al. Contemporary cefdinir activity against North American isolates from communityacquired urinary tract infections [abstract no. 34.013]. 11th International Congress on Infectious Diseases; 2004 Mar 4–7; Cancun
Livermore DM. Beta-lactamase-mediated resistance and opportunities for its control. J Antimicrob Chemother 1998; 41 Suppl. D: 25–41
Doern GV, Jones RN, Pfaller MA, et al. Haemophilus influenzae and Moraxella catarrhalis from patients with community-acquired respiratory tract infections: antimicrobial susceptibility patterns from the SENTRY Antimicrobial Program (United States and Canada, 1997). Antimicrob Agents Chemother 1999 Feb; 43(2): 385–9
Felmingham D, Gruneberg RN, Alexander Project Group. The Alexander Project 1996–1997: latest susceptibility data from this international study of bacterial pathogens from community-acquired lower respiratory tract infections. J Antimicrob Chemother 2000 Feb; 45(2): 191–203
Bootsma HJ, van Dijk J, Verhoef J, et al. Molecular characterization of the BRO beta-lactamase of Moraxella (Branhamella) catarrhalis. Antimicrob Agents Chemother 1996 Apr; 40(4): 966–72
Payne DJ, Amyes SG. The sensitivity of clinical bacteria isolated in Scotland to the oral cephalosporin, cefdinir. Drugs Exp Clin Res 1992; 18(6): 225–31
Jacoby GA, Carreras I. Activities of beta-lactam antibiotics against Escherichia coli strains producing extended-spectrum beta-lactamases. Antimicrob Agents Chemother 1990 May; 34(5): 858–62
Payne DJ, Amyes SG. Stability of cefdinir (C1-983, FK482) to extended-spectrum plasmid-mediated beta-lactamases. J Med Microbiol 1993 Feb; 38(2): 114–7
Labia R, Morand A. Interaction of cefdinir with betalactamases. Drugs Exp Clin Res 1994; 20(2): 43–8
Hatano K, Nishino T. Morphological alterations of Staphylococcus aureus and Streptococcus pyogenes exposed to cefdinir, a new oral broad-spectrum cephalosporin. Chemotherapy 1994 Mar–1994 30; 40(2): 73–9
Blandino G, Caccamo F, Di Marco R, et al. Bactericidal activity and postantibiotic effect of cefdinir (C1 983, FK 482) against selected pathogens. Drugs Exp Clin Res 1992; 18(8): 319–27
Yourassowsky E, Van der Linden MP, Crokaert F. Comparative kill and growth rates determined with cefdinir and cefaclor and with Streptococcus pneumoniae and beta-lactamase-producing Haemophilus influenzae. Antimicrob Agents Chemother 1992 Jan; 36(1): 46–9
Garcia-Rodriguez JA, Trujillano-Martin I, Garcia-Sanchez JE. Cefdinir: in vitro activity study and effect of human serum. Drugs Exp Clin Res 1993; 19(2): 51–8
Pruul H, McDonald PJ. Cefdinir-induced modification of the susceptibility of bacteria to the antibacterial activity of human serum and polymorphonuclear neutrophils. Eur J Clin Microbiol Infect Dis 1993 Mar; 12(3): 170–6
Guay DRP. Cefdinir: an advanced-generation, broad-spectrum oral cephalosporin. Clin Ther 2002 Apr; 24(4): 473–89
Craig WA. Interrelationship between pharmacokinetics and pharmacodynamics in determining dosage regimens for broad spectrum cephalosporins. Diagn Microbiol Infect Dis 1995 May–Jun; 22(1–2): 89–96
Craig WA. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 1998 Jan; 26(1): 1–10
Nicolau DP. Pharmacodynamic considerations in oral cephalosporin therapy: implications for selection and effectiveness. Infect Dis Clin Pract 1998; 7 (2 Suppl.): S81–5
Novelli A, Fallani S, Cassetta MI, et al. Pharmacokinetics and pharmacodynamics of oral cephalosporins as critical factors in choice of antibiotics. Int J Antimicrob Agents 2000; 16(4): 501–5
Fukuoka T, Kawada H, Kitayama A, et al. Efficacy of CS-834 against experimental pneumonia caused by penicillin-susceptible and -resistant Streptococcus pneumoniae in mice. Antimicrob Agents Chemother 1998 Jan; 42(1): 23–7
Tawara S, Hatano K, Wakai Y, et al. In vivo antibacterial activity of FK041, a new orally active cephalosporin. J Antibiot (Tokyo) 1999 Jul; 52(7): 660–5
Miyazaki S, Fujikawa T, Matsumoto T, et al. Efficacy of azithromycin, clarithromycin and beta-lactam agents against experimentally induced bronchopneumonia caused by Haemophilus influenzae in mice. J Antimicrob Chemother 2001 Sep; 48(3): 425–30
Guttendorf R, Koup J, Misiak P, et al. Pharmacokinetics of cefdinir (CI-983, FK-482) in children: NONMEM analysis, dose selection and body size factors [abstract no. 1227]. 32nd Interscience Conference on Antimicrobial Agents and Chemotherapy; 1992 Oct 11–14; Anaheim, 315
Richer M, Allard S, Manseau L, et al. Suction-induced blister fluid penetration of cefdinir in healthy volunteers following ascending oral doses. Antimicrob Agents Chemother 1995 May; 39(5): 1082–6
Misiak P, Guttendorf R, Vassos A, et al. Effect of high-fat meal on the pharmacokinetics of cefdinir (CI-983, FK-482) [abstract no. 963]. 32nd Interscience Conference on Antimicrobial Agents and Chemotherapy; 1992 Oct 11–14; Anaheim, 271
Hishida A, Ohishi K, Nagashima S, et al. Pharmacokinetic study of an oral cephalosporin, cefdinir, in hemodialysis patients. Antimicrob Agents Chemother 1998 Jul; 42(7): 1718–21
Lepsy CS, Guttendorf RJ, Kugler AR, et al. Effects of organic anion, organic cation, and dipeptide transport inhibitors on cefdinir in the isolated perfused rat kidney. Antimicrob Agents Chemother 2003 Feb; 47(2): 689–96
Cook PJ, Andrews JM, Wise R, et al. Distribution of cefdinir, a third generation cephalosporin antibiotic, in serum and pulmonary compartments. J Antimicrob Chemother 1996 Feb; 37(2): 331–9
Guay DRP. Pharmacodynamics and pharmacokinetics of cefdinir, an oral extended spectrum cephalosporin. Pediatr Infect Dis J 2000 Dec; 19 (12 Suppl.): S141–6
Ueno K, Tanaka K, Tsujimura K, et al. Impairment of cefdinir absorption by iron ion. Clin Pharmacol Ther 1993 Nov; 54(5): 473–5
Data on file. Abbott Park (IL): Abbott Laboratories, 2003 Oct
Jacolot A, Tod M, Petitjean O. Pharmacokinetic interaction between cefdinir and two angiotensin-converting enzyme inhibitors in rats. Antimicrob Agents Chemother 1996 Apr; 40(4): 979–82
Paster RZ, McAdoo MA, Keyserling CH, et al. A comparison of a five-day regimen of cefdinir with a seven-day regimen of loracarbef for the treatment of acute exacerbations of chronic bronchitis. Int J Clin Pract 2000 Jun; 54(5): 293–9
Drehobl M, Bianchi P, Keyserling CH, et al. Comparison of cefdinir and cefaclor in treatment of community-acquired pneumonia. Antimicrob Agents Chemother 1997 Jul; 41(7): 1579–83
Fogarty CM, Bettis RB, Griffin TJ, et al. Comparison of a 5 day regimen of cefdinir with a 10 day regimen of cefprozil for treatment of acute exacerbations of chronic bronchitis. J Antimicrob Chemother 2000 Jun; 45(6): 851–8
Van Herwaarden CL, Langan CE, Siemon G, et al. International study comparing cefdinir and cefuroxime axetil in the treatment of patients with acute exacerbation of chronic bronchitis. Int J Infect Dis 2000; 4(1): 26–33
Gwaltney Jr JM, Savolainen S, Rivas P, et al. Comparative effectiveness and safety of cefdinir and amoxicillin-clavulanate in treatment of acute community-acquired bacterial sinusitis: Cefdinir Sinusitis Study Group. Antimicrob Agents Chemother 1997 Jul; 41(7): 1517–20
Steurer M, Schenk P. Efficacy and safety of cefdinir in the treatment of maxillary sinusitis. Eur Arch Otorhinolaryngol 2000; 257(3): 140–8
Nemeth MA, McCarty J, Gooch 3rd WM, et al. Comparison of cefdinir and penicillin for the treatment of streptococcal pharyngitis: Cefdinir Pharyngitis Study Group. Clin Ther 1999 Nov; 21(11): 1873–81
Tack KJ, Henry DC, Gooch WM, et al. Five-day cefdinir treatment for streptococcal pharyngitis: Cefdinir Pharyngitis Study Group. Antimicrob Agents Chemother 1998 May; 42(5): 1073–5
Tack KJ, Littlejohn TW, Mailloux G, et al. Cefdinir versus cephalexin for the treatment of skin and skin-structure infections: the Cefdinir Adult Skin Infection Study Group. Clin Ther 1998 Mar–Apr 30; 20(2): 244–56
Adler M, McDonald PJ, Trostmann U, et al. Cefdinir vs. amoxicillin/clavulanic acid in the treatment of suppurative acute otitis media in children. Pediatr Infect Dis J 2000 Dec; 19 (12 Suppl.): S166–70
Block SL, McCarty JM, Hedrick JA, et al. Comparative safety and efficacy of cefdinir vs amoxicillin/clavulanate for treatment of suppurative acute otitis media in children. Pediatr Infect Dis J 2000 Dec; 19 (12 Suppl.): S159–65
Block SL, Kratzer J, Nemeth MA, et al. Five-day cefdinir course vs. ten-day cefprozil course for treatment of acute otitis media. Pediatr Infect Dis J 2000 Dec; 19 (12 Suppl.): S147–52
Nemeth MA, Gooch 3rd WM, Hedrick J, et al. Comparison of cefdinir and penicillin for the treatment of pediatric streptococcal pharyngitis. Clin Ther 1999 Sep; 21(9): 1525–32
Tack KJ, Hedrick JA, Rothstein E, et al. A study of 5-day cefdinir treatment for streptococcal pharyngitis in children: Cefdinir Pediatric Pharyngitis Study Group. Arch Pediatr Adolesc Med 1997 Jan; 151(1): 45–9
Tack KJ, Keyserling CH, McCarty J, et al. Study of use of cefdinir versus cephalexin for treatment of skin infections in pediatric patients: the Cefdinir Pediatric Skin Infection Study Group. Antimicrob Agents Chemother 1997 Apr; 41(4): 739–42
Block SL, Harrison CJ, Hedrick JA, et al. Penicillin-resistant Streptococcus pneumoniae in acute otitis media: risk factors, susceptibility patterns and antimicrobial management. Pediatr Infect Dis J 1995 Sep; 14(9): 751–9
Klein JO. Otitis media in infants and children. Infect Dis Clin Pract 2000; 9(8): 319–22
Easton J, Noble S, Perry CM, et al. Amoxicillin/clavulanic acid: a review of its use in the management of paediatric patients with acute otitis media. Drugs 2003; 63(3): 311–40
Hoberman A, Paradise JL. Duration of therapy for acute otitis media. Pediatr Infect Dis J 2000 May; 19(5): 471–3
Block S, Busman T, Paris M, et al. Five-day regimen of cefdinir is similar to ten-day regimen of amoxicillin/clavulanate in the treatment of acute otitis media [poster]. 4th Pediatric Infectious Disease Society Conference; 2003 Oct 12–14; Rancho Bernado
Pichichero ME, Gooch 3rd WM. Comparison of cefdinir and penicillin V in the treatment of pediatric streptococcal tonsillopharyngitis. Pediatr Infect Dis J 2000 Dec; 19 (12 Suppl.): S171–3
Steele RW, Thomas MP, Begue RE. Compliance issues related to the selection of antibiotic suspensions for children. Pediatr Infect Dis J 2001 Jan; 20(1): 1–5
Steele RW, Blumer JL, Kalish GH. Patient, physician, and nurse satisfaction with antibiotics. Clin Pediatr (Phila) 2002 Jun; 41(5): 285–99
Powers JL, Gooch 3rd WM, Oddo LP. Comparison of the palatability of the oral suspension of cefdinir vs. amoxicillin/ clavulanate potassium, cefprozil and azithromycin in pediatric patients. Pediatr Infect Dis J 2000 Dec; 19 (12 Suppl.): S174–80
Leibovitz E. Acute otitis media in pediatric medicine: current issues in epidemiology, diagnosis and management. Pediatric Drugs 2003; 5 Suppl. 1: 1–12
Ramgoolam A, Steele R. Formulations of antibiotics for children in primary care: effects on compliance and efficacy. Paediatr Drugs 2002; 4(5): 323–33
Cefdinir prescribing information. Bethesda (MD): American Hospital Formulary Service Drug Information, 2003: 147–9
Bergogne-Berezin E. Oral cephalosporins in the treatment of respiratory tract infections. Cur Ther Res, Clin & Exp 1996; 57 Suppl. A: 87–96
Birnbaumer D, Fernandez-Frackelton M. The new antibiotics. Emerg Med Clin North Am 2000; 18(4): 671–708
Felmingham D. Review of the comparative in vitro activity of some oral cephalosporins. Infect Dis Clin Pract 1998 Aug; 7 Suppl. 2: 75–80
Doern GV. Antimicrobial use and the emergence of antimicrobial resistance with Streptococcus pneumoniae in the United States. Clin Infect Dis 2001; 33 Suppl. 3: S187–92
Jones RN, Jenkins SG, Hoban DJ, et al. In vitro activity of selected cephalosporins and erythromycin against staphylococci and pneumococci isolated at 38 North American medical centers participating in the SENTRY Antimicrobial Surveillance Program, 1997–1998. Diagn Microbiol Infect Dis 2000 Jun; 37(2): 93–8
Doern GV. Antimicrobial resistance with Streptococcus pneumoniae in the United States. Sem Resp Crit Care Med 2000; 21(4): 273–84
Hoban DJ, Doern GV, Fluit AC, et al. Worldwide prevalence of antimicrobial resistance in Streptococcus penumoniae, Haemophilus influenzae, and Moraxella catarrhalis in the SENTRY antimicrobial surveillance program, 1997–1999. Clin Infect Dis 2001 May 15; 32 Suppl. 2: S81–93
Sader HS, Gales AC, Granacher TD, et al. Prevalence of antimicrobial resistance among respiratory tract isolates in Latin America: results from SENTRY antimicrobial surveillance program (1997–98). Brazilian J Infect Dis 2000 Oct; 4(5): 245–54
Doern GV. Antimicrobial resistance with Streptococcus pneumoniae: much ado about nothing? Semin Respir Infect 2001 Sep; 16(3): 177–85
Antimicrobial treatment guidelines for acute bacterial rhinosinusitis (ABRS). Washington, DC: Sinus and Allergy Health Partnership, 2004
Pichichero ME, Reiner SA, Brook I, et al. Controversies in the medical management of persistent and recurrent acute otitis media: recommendations of a clinical advisory committee. Ann Otol Rhinol Laryngol Suppl 2000 Aug; 183: 1–12
McCracken Jr GH. Prescribing antimicrobial agents for treatment of acute otitis media. Pediatr Infect Dis J 1999 Dec; 18(12): 1141–6
Klein JO. Review of consensus reports on management of acute otitis media. Pediatr Infect Dis J 1999 Dec; 18(12): 1152–5
Blumer JL. Fundamental basis for rational therapeutics in acute otitis media. Pediatr Infect Dis J 1999 Dec; 18(12): 1130–40
Leibovitz E, Dagan R. Antibiotic treatment for acute otitis media. Int J Antimicrob Agents 2000 Aug; 15(3): 169–77
Dowell S, Butler J, Giebink G, et al. Acute otitis media: management and surveillance in an era of pneumococcal resistance: a report from the Drug-resistant Streptococcus pneumoniae Therapeutic Working Group. Pediatr Infect Dis 1999 Jan; 18(1): 1–9
Erramouspe J, Heyneman CA. Treatment and prevention of otitis media. Ann Pharmacother 2000 Dec; 34(12): 1452–68
Hoberman A, Marchant CD, Kaplan SL, et al. Treatment of acute otitis media consensus recommendations. Clin Pediatr (Phila) 2002 Jul–Aug; 41(6): 373–90
American Academy of Pediatrics and American Academy of Family Physicians. Subcommittee on Management of Acute Otitis Media. Clinical practice guideline: diagnosis and management of acute otitis media [online]; http://www.aap.org/ policy/aomfinal [Accessed 2004 Mar 9].
Bisno AL, Gerber MA, Gwaltney Jr JM, et al. Practice guidelines for the diagnosis and management of group A streptococcal pharyngitis: Infectious Diseases Society of America. Clin Infect Dis 2002 Jul 15; 35(2): 113–25
Brook I. Failure of penicillin to eradicate group A beta-hemolytic streptococci tonsillitis: causes and management. J Otolaryngol 2001 Dec; 30(6): 324–9
Pichichero ME. Streptococcal tonsillopharyngitis: advantages of shorter antibiotic courses. Infect Med 2001; 18(11): 515–8
Brook I. Antibacterial therapy for acute group A streptococcal pharyngotonsillitis: short-course versus traditional 10-day oral regimens. Paediatr Drugs 2002; 4(11): 747–54
Stevens DL. Current concepts in the treatment of streptococcal pharyngitis. Infect Dis Clin Pract 1998 Aug; 7 Suppl. 2: 86–9
Guay DRP. Short-course antimicrobial therapy for upper respiratory tract infections. Clin Ther 2000 Jun; 22(6): 673–84
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: D.J. Biedenbach, The JONES Group, JMI Laboratories, North Liberty, Iowa, USA; S.L. Block, Physicians to Children & Adolescents, Bardstown, Kentucky, USA; P.J. Cook, Department of Respiratory Medicine, Dryburn Hospital, Durham, England; W.M. Gooch III, University of Utah School of Medicine, Salt Lake City, Utah, USA; J.M. Gwaltney Jr, Department of Internal Medicine, University of Virginia Health Science Center, Charlottesville, Virginia, USA; P.J. McDonald, Department of Clinical Microbiology, Flinders University of South Australia, Bedford Park, South Australia, Australia; M.E. Pichichero, Department of Microbiology and Immunology, School of Medicine and Dentistry, University of Rochester, Rochester, New York, USA.
Data Selection
Sources: Medical literature published in any language since 1980 on cefdinir, identified using Medline and EMBASE, supplemented by AdisBase (a proprietary database of Adis International). Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: Medline search terms were ‘cefdinir’ or ‘BMY-28488’ or ‘CI-983’ or ‘FK-482’ or ‘PD-134393’. EMBASE search terms were ‘cefdinir’. AdisBase search terms were ‘cefdinir’ or ‘CI-983’ or ‘FK-482’ or ‘PD-134393’. Searches were last updated 8 June 2004.
Selection: Studies in patients with bacterial infections who received cefdinir. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Cefdinir, pharmacodynamics, pharmacokinetics, therapeutic use.
Rights and permissions
About this article
Cite this article
Perry, C.M., Scott, L.J. Cefdinir. Drugs 64, 1433–1464 (2004). https://doi.org/10.2165/00003495-200464130-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200464130-00004