Summary
Abstract
The fixed-dose combination of enalapril 10mg with nitrendipine 20mg combines an ACE inhibitor with a calcium channel antagonist (CCA) and is indicated for the treatment of patients with mild-to-moderate hypertension whose blood pressure (BP) is inadequately controlled with enalapril or nitrendipine monotherapy. In randomised, double-blind clinical trials, enalapril/nitrendipine 10/20 mg/day was significantly more effective than its individual components in reducing diastolic BP (DBP) in patients with mild-to-moderate hypertension inadequately controlled with enalapril 10 mg/day or nitrendipine 20 mg/day. The fixed-dose combination was similar in efficacy at reducing DBP to amlodipine 10 mg/day in patients who failed to achieve BP control with amlodipine 5 mg/day, and to losartan/hydrochlo-rothiazide 50/12.5 mg/day in patients who received the combinations as first-line therapy. Enalapril/nitrendipine 10/20mg produced a consistent antihypertensive effect that persisted for the entire 24-hour dosage interval as shown by ambulatory BP monitoring.
Enalapril/nitrendipine 10/20mg was well tolerated in clinical trials where it was administered to patients with mild-to-moderate hypertension for up to 12 weeks. The adverse events were those expected of ACE inhibitors and CCAs and included cough, headache and flushing. Evidence from clinical trials, including a pooled analysis, suggests that the incidence of oedema may be significantly lower with the fixed-dose combination than with CCA monotherapy.
In conclusion, enalapril/nitrendipine 10/20mg is a well tolerated fixed-dose combination of two established antihypertensive agents administered once daily that effectively lowers BP throughout the 24-hour dosage interval. Importantly, the fixed-dose combination may have a lower incidence of oedema than CCA monotherapy. Enalapril/nitrendipine 10/20mg provides an additional treatment option for patients with mild-to-moderate hypertension for whom combination therapy is appropriate..
Pharmacodynamic Properties
Enalapril is an ACE inhibitor prodrug that is converted to the active moiety enalaprilat after absorption from the gastrointestinal tract. Nitrendipine is a dihydropyridine calcium channel antagonist (CCA).
In patients with hypertension, the single agents enalapril and nitrendipine as well as their combination effectively lower BP on a once-daily basis. Both have long-term beneficial effects on the cardiovascular system, improving left ventricular structure and function, with neutral and/or moderating effects on renal function, metabolic parameters and insulin sensitivity. Neither of these agents has any significant effect on electrolyte balance.
Enalapril/nitrendipine combination did not have any significant effect on heart rate (HR) in healthy volunteers..
Pharmacokinetic Properties
There were no clinically relevant differences in the area under the plasma concentration-time curve and maximum plasma concentration values of enalapril or nitrendipine after single-dose administration of the fixed-dose combination enalapril/nitrendipine 10/20mg, compared with those observed with single agent enalapril 10mg or nitrendipine 20mg. There was no other pharmacokinetic interaction between the two drugs when coadministered..
Therapeutic Efficacy
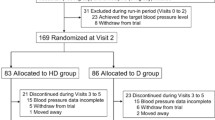
In two randomised, double-blind studies, patients with mild-to-moderate hypertension whose BP was not controlled with enalapril 10 mg/day or nitrendipine 20 mg/day monotherapies had significantly greater reductions in diastolic BP (DBP) with enalapril/nitrendipine 10/20 mg/day than those who continued on their respective monotherapies for 12 weeks. Pooled analysis of these two trials showed that the reduction in both systolic BP (SBP) and DBP with the combination therapy was significantly higher than that in patients receiving monotherapy (enalapril or nitrendipine). BP response rates were also significantly higher with enalapril/nitrendipine than those seen with the monotherapies in the pooled analysis, although one of the randomised trials did not show statistically significant differences between groups for SBP and DBP response rates.
After failure of amlodipine 5 mg/day to achieve BP control in patients with mild-to-moderate hypertension, enalapril/nitrendipine 10/20 mg/day was as effective as doubling the dosage of amlodipine in reducing DBP after 6 weeks. Although amlodipine 10 mg/day achieved significantly greater SBP reduction, there was no significant difference between the two treatments with respect to the proportions of patients achieving control of BP.
Enalapril/nitrendipine 10/20 mg/day was generally similar to a fixed-dose combination of losartan/hydrochlorothiazide (HCTZ) 50/12.5 mg/day in reducing BP in patients with mild-to-moderate hypertension as indicated by the reductions in office, 24-hour, daytime or night-time SBP/DBP measured by ambulatory BP monitoring (ABPM). Response rates were high and similar for both treatments. The only significant difference between the two treatments was with respect to office SBP control rates in favour of enalapril/nitrendipine.
The values of mean trough-to-peak ratios and the smoothness indices for the two treatments estimated during ABPM showed that the antihypertensive effects of both treatments persisted for the entire 24-hour dosage interval.
Pharmacoeconomic analyses of enalapril/nitrendipine therapy are limited to a modelled cost-effectiveness analysis conducted in Spain. Results indicated that enalapril/nitrendipine may provide a cost-effective treatment option in the treatment of hypertension.
Tolerability
Enalapril/nitrendipine 10/20 mg/day was well tolerated in clinical trials, with the well known adverse effects of ACE inhibitors (e.g. cough) or CCAs (e.g. oedema, flushing and headache) being the most commonly reported adverse events.
The combination was at least as well tolerated as its individual components, and better tolerated than amlodipine 10 mg/day as shown by the incidence of adverse events considered to be at least possibly treatment-related, especially that of oedema. The incidence of oedema associated with enalapril/nitrendipine combination was two to three times lower than that observed with either nitrendipine 20 mg/day or amlodipine 10 mg/day. Adverse events observed with enalapril/ nitrendipine were generally similar in nature and incidence to those seen with losartan/HCTZ 50/12.5 mg/day.
Enalapril/nitrendipine had a neutral or a mild positive effect on the HR in clinical trials in patients with mild-to-moderate hypertension.
Adverse events were an infrequent cause of discontinuation of treatment with enalapril/nitrendipine in clinical trials. No deaths or serious adverse events related to study medication were reported in any of the trials.






Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Materson BJ, Reda DJ, Cushman WC, et al. Single-drag therapy for hypertension in men: a comparison of six anti-hypertensive agents with placebo. The Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. N Engl J Med 1993; 328(13): 914–21
Sever P. The heterogeneity of hypertension: why doesn’t every patient respond to every antihypertensive drag? J Hum Hypertens 1995; 9 Suppl. 2: S33–6
Epstein M, Waeber B. Fixed-dose combination therapy with calcium antagonists. In: Epstein M, editor. Calcium antagonists in clinical medicine. 3rd ed. Philadelphia (PA): Hanley and Belfus, Inc., 2002: 713–30
Schmieder RE, Schlaich MP, Klingbeil AU, et al. Update on reversal of left ventricular hypertrophy in essential hypertension (a meta-analysis of all randomised double-blind studies until December 1996). Nephrol Dial Transplant 1998; 13(3): 564–9
Messerli FH. Combinations in the treatment of hypertension: ACE inhibitors and calcium antagonists. Am J Hypertens 1999; 12 (8 Pt 2): 86S–90S
Weir MR. When antihypertensive monotherapy fails: fixed-dose combination therapy. South Med J 2000; 93(6): 548–56
Mènard J, Bellet M. Calcium antagonists-ACE inhibitors combination therapy: objectives and methodology of clinical development. J Cardiovasc Pharmacol 1993; 21 Suppl. 2: S49–54
Zanchetti A. Nitrendipine and ACE inhibitors. J Cardiovasc Pharmacol 1988; 12 Suppl. 4: S80–5
Todd PA, Goa KL. Enalapril: a reappraisal of its pharmacology and therapeutic use in hypertension. Drags 1992 Mar; 43(3): 346–81
Goa KL, Sorkin EM. Nitrendipine: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in the treatment of hypertension. Drugs 1987 Feb; 33(2): 123–55
Wilde MI, Bryson HM, Goa KL. Enalapril: a review of quality-of-life and pharmacoeconomic aspects of its use in heart failure and mild to moderate hypertension. Pharmacoeconomics 1994 Aug; 6(2): 155–82
Scriabine A, Vanov S, Deck K. Nitrendipine. Baltimore (MD): Urban and Schwarzenberg, 1984
Santiago TM, Lopez LM. Nitrendipine, a new dihydropyridine calcium-channel antagonist for the treatment of hypertension. Drug Intell Clin Pharm 1990; 24(2): 167–75
Picca M, Bisceglia J, Zocca A, et al. Effects of enalapril and amlodipine on left ventricular hypertrophy and function in essential hypertension. Clin Drug Invest 1997; 13 Suppl. 1: 29–35
Cuspidi C, Muiesan ML, Valagussa L, et al. Comparative effects of candesartan and enalapril on left ventricular hypertrophy in patients with essential hypertension: the candesartan assessment in the treatment of cardiac hypertrophy (CATCH) study. J Hypertens 2002; 20(11): 2293–300
Devereux RB,Palmieri V, Sharpe N, et al. Effects of once-daily angiotensin-converting enzyme inhibition and calcium channel blockade-based antihypertensive treatment regimens on left ventricular hypertrophy and diastolic filling in hypertension: the prospective randomized enalapril study evaluating regression of ventricular enlargement (PRESERVE) trial. Circulation 2001; 104(11): 1248–54
Rockstroh JK, Schobel HP, Vogt-Ladner G, et al. Blood pressure independent effects of nitrendipine on cardiac structure in patients after renal transplantation. Nephrol Dial Transplant 1997; 12(7): 1441–7
Matsumoto M, Deng YB, Munehira J, et al. Effects of nitrendipine on left ventricular structure and function and aortic distensibility in elderly patients with isolated systolic hypertension. Curr Ther Res Clin Exp 1997 Feb; 58(2): 117–26
Modena MG, Mattioli AV, Parato VM, et al. Effect of antihypertensive treatment with nitrendipine on left ventricular mass and diastolic filling in patients with mild to moderate hypertension. J Cardiovasc Pharmacol 1992 Jan; 19(1): 148–53
Gerritsen TA, Bak AAA, Stolk RP, et al. Effects of nitrendipine and enalapril on left ventricular mass in patients with non-insulin-dependent diabetes mellitus and hypertension. J Hypertens 1998 May; 16(5): 689–96
Mosconi L, Ruggenenti P, Perna A, et al. Nitrendipine and enalapril improve albuminuria and glomeralar filtration rate in non-insulin dependent diabetes. Kidney Int 1996 Jun; 49 Suppl. 55: S91–3
Ross SA, Josefsberg Z, Fick GH, et al. Effects of nitrendipine and enalapril in hypertensive type II diabetic subjects with microalbuminuria. J Hypertens 1992 Jun; 10 Suppl. 4: 310
Estacio RO, Jeffers BW, Gifford N, et al. Effect of blood pressure control on diabetic microvascular complications in patients with hypertension and type 2 diabetes. Diabetes Care 2000; 23 Suppl. 2: B54–64
Scaglione R, Indovina A, Parrinello G, et al. Antihypertensive efficacy and effects of nitrendipine on cardiac and renal hemodynamics in mild to moderate hypertensive patients: randomized controlled trial versus hydrochlorothiazide. Cardiovasc Drugs Ther 1992 Apr; 6(2): 141–6
Pinol C, Cobos A, Cases A, et al. Nitrendipine and enalapril in the treatment of diabetic hypertensive patients with microalbuminuria. Kidney Int 1996 Jun; 49 Suppl. 55: S85–7
Ruggenenti P, Mosconi L, Bianchi L, et al. Long-term treatment with either enalapril or nitrendipine stabilizes albuminuria and increases glomerular filtration rate in non-insulin-dependent diabetic patients. Am J Kidney Dis 1994 Nov; 24(5): 753–61
Lender D, Arauz-Pacheco C, Breen L, et al. A double blind comparison of the effects of amlodipine and enalapril on insulin sensitivity in hypertensive patients. Am J Hypertens 1999; 12(3): 298–303
Kazda S, Hirth C, Stasch JP. Diuretic effect of nitrendipine contributes to its antihypertensive efficacy: a review. J Cardiovasc Pharmacol 1988; 12 Suppl. 4: S1–5
Ruilope LM. Renal effects of nitrendipine. J Cardiovasc Pharmacol 1991; 18 Suppl. 5: S10–3
Paolisso G, Aceto E, Cennamo G, et al. Metabolic effects of nitrendipine. Clin Ther 1991; 13(6): 695–8
Mancini M, Marotta T, Ferrara LA. Metabolic neutrality in nitrendipine therapy. J Cardiovasc Pharmacol 1991; 18 Suppl. 1: S30–3
Frontera G, Delgadillo J, Calvo G, et al. Pharmacokinetic interaction study between enalapril and nitrendipine in healthy subjects [abstract no. 395]. Eur J Clin Pharmacol 1997; 52 Suppl.: A131
Todd PA, Goa KL. Enalapril: an update of its pharmacological properties and therapeutic use in congestive heart failure. Drugs 1989 Feb; 37(2): 141–61
Grupo-Vita. Development of a fixed dose combination of enalapril/nitrendipine for the treatment of arterial hypertension. Barcelona: Grupo-Vita, 2003. (Data on file)
Committee for Proprietary Medicinal Products. Note for guidance on the investigation of bioavailability and bioequivalence of the European Agency for the Evaluation of Medicinal Products (CPMP/EWP/QWP/1401/98) [online]. Available from URL: http://www.emea.eu.int [Accessed 2004 Apr 15]
Roca-Cusachs Aygrupoespañoldeestudioenalapril/nitrendipino. Evaluación de la eficacia de la asociación de Enalapril 10/ Nitrendipino 20 en pacientes que no se controlan con monoterapia de enalapril o nitrendipino [in Spanish; abstract no. 85T]. Hipertensión 2002; 19 Suppl. 2: 175. Plus poster presented at the 7a Reuníón Nacíonal Sociedad Española de Hípertensíón Líga Española para la Lucha contra la Hipertensíón Arterial; 2002 Mar 12–15; Madrid
Forette F, Seux ML, Staessen JA, et al. Prevention of dementia in randomised double-blind placebo-controlled systolic hypertension in Europe (Syst-Eur) trial. Lancet 1998; 352(9137): 1347–51.
de la Sierra A, Gil-Extremera B, Calvo C, et al. Comparison of the antihypertensive effects of the fixed dose combination enalapril 10 mg / nitrendipine 20 mg versus losartan 50 mg / hydrochlorothiazide 12.5 mg, assessed by 24-hour ambulatory blood pressure monitoring, in essential hypertensive patients. J Hum Hypertens 2004; 18(3): 215–22
Marin R, de la Sierra A, Roca-Cusachs A, et al. Comparison of a fixed-dose combination vs dose titration in second line therapy of hypertension [abstract no. P-436]. Am J Hypertens 2003 May; 16 (5 Pt 2): 195A. Plus poster presented at the 18th Annual Scientific Meeting of the American Society of Hypertension; 2003 May 14–17; New York
Anabile G, Bory M, Ledoux L, et al. Comparison of nitrendipine + enalapril and hydrochlorothiazide + enalapril in patients with enalapril resistant hypertension. J Hypertens 1992 Jun; 10 Suppl. 4: 177
Staessen JA, Fagard R, Thijs L, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. Lancet 1997 Sep 13; 350(9080): 757–64
Verkaaik R, Hoogewind R, Woittiez AJJ, et al. Effects of nitrendipine and enalapril in essential hypertension [abstract no. 123]. Neth J Med 1991 Dec; 39: A70
Gil-Extremera B, Spanish Group for Study of Enalapril/Ni-trendipine. ABPM Study: efficacy of two fixed dose combinations, enalapril/nitrendipine vs. losartan/hidrochlorotiazide, in not controlled mild-moderate hypertensive patients [abstract no. P-213]. Am J Hypertens 2003 May; 16 (5 Pt 2): 116. Plus poster presented at the 18th Annual Scientific Meeting of the American Society of Hypertension; 2003 May 14–17; New York
Roca-Cusachs A, Torres F, Horas M, et al. Nitrendipine and enalapril combination therapy in mild to moderate hypertension: assessment of dose-response relationship by a clinical trial of factorial design. J Cardiovasc Pharmacol 2001 Dec; 38(6): 840–9
Joint National Committee on Prevention DEaToHBP. The sixth report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure. Arch Intern Med 1997; 157(21): 2413–46
Roca-Cusachs A, Spanish Group for Study of enalapril/Ni-trendipine. Efficacy of a fixed dose combination of enalapril/ nitrendipine in patients not controlled with enalapril or nitrendipine monotherapy, results of pooled analysis of two studies: ENEAS-1 and ENEAS-2 [abstract no. P-41]. Am J Hypertens 2002 Apr; 15 (4 Pt 2): 48
Roca-Cusachs A, Pontes C, Combalia J, et al. Reduced incidence of oedema with the enalapril/nitrendipine fixed dose combination [abstract no. P2.205]. J Hypertension 2003; 21 Suppl. 4: S180. Plus poster presented at the 13th European Meeting on Hypertension; 2003 June 13–17; Milan
Grupo-Vita. Product information: Eneas® (enalapril maleate/ nitrendipine) tablets, 10/20mg. Barcelona: Grupo-Vita, 2003
Joint Formulary Committee. British National Formulary. 47th ed. London: British Medical Association and Royal Pharmaceutical Society of Great Britain [online]. Available from URL: http://www.bnf.org/ [Accessed 2004 Apr 15]
Merck Sharp & Dohme Limited. Product information: Innovace (enalapril) tablets, 2.5, 5, 10 and 20 mg [online]. Available from URL: http://emc.medicines.org.uk [Accessed 2004 Apr 15]
Kirch W, Hutt HJ, Heidemann H, et al. Drug interactions with nitrendipine. J Cardiovasc Pharmacol 1984; 6 Suppl. 7: S982–5
Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure. Hypertension 2003; 42(6): 1206–52
Guidelines Committee. 2003 European Society of Hypertension — European Society of Cardiology guidelines for the managment of arterial hypertension. J Hypertens 2003; 21(6): 1011–53
Williams B, Poulter NR, Brown MJ, et al. Guidelines for management of hypertension: report of the fourth working party of the British Hypertension Society, 2004 — BHS IV. J Hum Hypertens 2004; 18(3): 139–85
World Health Organization-International Society of Hypertension Writing Group. 2003 World Health Organization (WHO)/ International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens 2003; 21(11): 1983–92
The World Health Report 2002. Reducing Risks, Promoting Healthy Life. Geneva: The World Health Organization [online]. Available from URL: http://www.who.int [Accessed 2004 Jan 26]
Carretero O, Oparil S. Essential hypertension, part II: treatment. Circulation 2000; 101(4): 446–53
Zanchetti A. Contribution of fixed low-dose combinations to initial therapy in hypertension. Eur Heart J Suppl 1999; 1 Suppl. L: L5–9
Law MR, Wald NJ, Morris JK, et al. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ 2003; 326(7404): 1427
Sica DA. Rationale for fixed-dose combinations in the treatment of hypertension: the cycle repeats. Drugs 2002; 62(3): 443–62
Epstein M, Bakris G. Newer approaches to antihypertensive therapy. Use of fixed-dose combination therapy. Arch Int Med 1996; 156(17): 1969–78
Hosie J, Wiklund I. Managing hypertension in general practice: can we do better? J Hum Hypertens 1995; 9 Suppl. 2: S15–8
Graves JW. Management of difficult to control hypertension. Mayo Clin Proc 2000; 75(3): 278–84
Guidelines Subcommittee. 1999 World Health Organization —International Society of Hypertension Guidelines for the Management of Hypertension. Blood Press 1999; 8 Suppl. 1: 9–43
Berlowitz DR, Ash AS, Hickey EC, et al. Inadequate management of blood pressure in a hypertensive population. New Eng J Med 1998; 339(27): 1957–63
Carvajal A, Jabary N, Garcia Del Pozo J, et al. Fixed-dose combination vs monotherapy in first-line treatment of hypertension: a meta analysis [abstract no. P1030]. J Hypertension 2002; 20 Suppl. 4: S244. Plus poster presented at the Joint Meeting of the 19th Scientific Meeting of the International Society of Hypertension and the 12th Meeting of the European Society of Hypertension; 2002 June 23–27; Prague
Antoñanzas F, Velasco M, Abbas I, et al. Modelo teórico de análisis coste-efectividad de la terapia combinada de enalapril-nitrendipino en el tratamiento de la hipertensión arterial [in Spanish]. Aten Primaria 2003; 31(6): 366–71
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: F. Antoñanzas, Departamento de Economía y Empresa, Universidad de la Rioja, Logroño, Spain; A. de la Sierra, Hypertension Unit, Department of Internal Medicine, Hospital Clínic, Barcelona, Spain; P.W. de Leeuw, Department of Medicine, University Hospital, Universiteit Maastricht, Maastricht, The Netherlands; R. Fagard, Hypertension and Cardiovascular Rehabilitation Unit, Katholieke Universiteit Leuven, Leuven, Belgium; G. Leonetti, Istituto di Medicina Cardiovascolare, Università degli Studi di Milano, Milan, Italy; R. Marín, Hospital de Covadonga, Oviedo, Spain; G.T. McInnes, Division of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow, United Kingdom; A. Roca-Cusachs, Department of Internal Medicine, Hospital de la Santa Creu i St Pau, Universitat Autonoma de Barcelona, Barcelona, Spain; J. Wang, Hypertension and Cardiovascular Rehabilitation Unit, Katholieke Universiteit Leuven, Leuven, Belgium; J. Webster, Department of Pharmacy, Grampian Health Board, Aberdeen, United Kingdom; A. Zanchetti, Centro di Fisiologia Clinica e Ipertensione, Università degli Studi di Milano, Milan, Italy.
Data Selection
Sources: Medical literature published in any language since 1980 on enalapril/nitrendipine, identified using Medline and EMBASE, supplemented by AdisBase (a proprietary database of Adis International). Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: Medline search terms were ‘enalapril nitrendipine’ or ‘enalapril’ or ‘nitrendipine’. EMBASE search terms were ‘enalapril nitrendipine’ or ‘enalapril’ or ‘nitrendipine’. AdisBase search terms were ‘enalapril nitrendipine’ or ‘enalapril’ or ‘nitrendipine’. Searches were last updated 20 April 2004.
Selection: Studies in patients with essential hypertension who received enalapril/nitrendipine. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Enalapril/nitrendipine, hypertension, pharmacodynamics, pharmacokinetics, therapeutic use, adverse events.
Rights and permissions
About this article
Cite this article
Siddiqui, M.A.A., Plosker, G.L. Fixed-Dose Combination Enalapril/Nitrendipine. Drugs 64, 1135–1148 (2004). https://doi.org/10.2165/00003495-200464100-00009
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200464100-00009