Abstract
-
▴ Enfuvirtide is the first of a new class of drugs, the fusion inhibitors. It is a synthetic peptide which binds to the HIV glycoprotein 41 (gp41), blocking fusion of the viral and cellular membranes.
-
▴ HIV isolates with reduced susceptibility to enfuvirtide have been recovered from patients receiving enfuvirtide in combination with other antiretroviral agents.
-
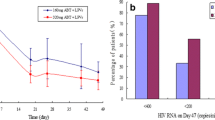
▴ Enfuvirtide 90mg (subcutaneously, twice daily) in combination with optimised background (OB) antiretroviral therapy significantly reduced plasma HIV RNA levels compared with OB alone after treatment for 24 weeks in two randomised trials involving adults with advanced HIV infection. The antiviral efficacy of enfuvirtide was maintained through to 48 weeks.
-
▴ At 24 and 48 weeks, the increase from baseline in the CD4+ cell count was significantly greater for patients receiving enfuvirtide plus OB than for those receiving OB alone.
-
▴ Enfuvirtide 30 mg/m2 or 60 mg/m2 in combination with other antiretroviral agents reduced plasma HIV RNA levels and increased CD4+ cell counts in a small trial involving paediatric patients with HIV infection.
-
▴ Local injection-site reactions were common. Lymphadenopathy and pneumonia occurred more often in patients receiving enfuvirtide plus OB than in the control group. The incidence of most other events was similar in each group.


Similar content being viewed by others
Notes
Use of a tradenames is for product identification purposes only and does not imply endorsement.
References
Kilby JM, Hopkins S, Venetta TM, et al. Potent suppression of HIV-1 replication in humans by T-20, a peptide inhibitor of gp41-mediated virus entry. Nat Med 1998 Nov; 4(11): 1302–7
Vandamme AM, Van Laethem K, De Clerq E. Managing resistance to anti-HIV drugs: an important consideration for effective disease management. Drugs 1999 Mar; 57(3): 337–61
World Health Organization. Scaling up antiretroviral therapy in resource-limited settings: guidelines for a public health approach [online]. Available from URL: http://www.who.int/hiv/topics/arv/ISBN9241545674.pdf [Accessed 2003 Jun 1]
Yeni PG, Hammer SM, Carpenter CCJ, et al. Antiretroviral treatment for adult HIV infection in 2002: updated recommendations of the International AIDS Society-USA Panel. JAMA 2002 Jul 10; 288(2): 222–35
Kilby JM. Therapeutic potential of blocking HIV entry into cells: focus on membrane fusion inhibitors. Expert Opin Invest Drugs 1999 Aug; 8(8): 1157–70
Kilby JM, Lalezari JP, Eron JJ, et al. The safety, plasma pharmacokinetics, and antiviral activity of subcutaneous enfuvirtide (T-20), a peptide inhibitor of gp41-mediated virus fusion, in HIV-infected adults [published erratum appears in AIDS Res Hum Retroviruses 2003 Jan 1; 19 (1): 83]. AIDS Res Hum Retroviruses 2002 Jul 1; 18(10): 685–93
De Clercq E. The emerging role of fusion inhibitors in HIV infection. Drugs R&D 1999 Nov; 2(5): 321–31
Adis R&D profiles: Pentafuside: DP 178, T 20. Drugs RD 1999 Nov; 2(5): 347–9
Chen RY, Kilby JM, Saag MS. Enfuvirtide. Expert Opin Investig Drugs 2002 Dec; 11(12): 1837–43
Cammack N. The potential for HIV fusion inhibition. Curr Opin Infect Dis 2001 Feb; 14(1): 13–6
Wild CT, Shugars DC, Greenwell TK, et al. Peptides corresponding to a predictive alpha-helical domain of human immunodeficiency virus type 1 gp41 are potent inhibitors of virus infection. Proc Natl Acad Sci USA 1994 Oct 11; 91(21): 9770–4
Roche Laboratories Inc., Trimeris Inc.. Fuzeon™ (enfuvirtide) for injection — Complete Product Information (US) [online]. Available from URL: http://www.rocheusa.com/products/fuzeon/pi.pdf [Accessed 2003 Apr 1]
Derdeyn CA, Decker JM, Sfakianos JN, et al. Sensitivity of human immunodeficiency virus type 1 to the fusion inhibitor T-20 is modulated by coreceptor specificity defined by the V3 loop of gpl 20. J Virol 2000 Sep; 74(18): 8358–67
Barney S, Guthrie K, Davis D, et al. Pentafuside (T20), a novel inhibitor of HIV-1 fusion and infection, is synergistic when used in combination with reverse transcriptase and protease inhibitors in vitro. Antiviral Res 1998 Mar; 37(3): A54
Greenberg ML, McDanal CB, Stanfield-Oakley SA. Virus sensitivity to T-20 and T-1249 is independent of coreceptor usage [abstract no. 473]. 8th Conference on Retroviruses and Opportunistic Infections; 2001 Feb 4–8; Chicago
Greenberg ML, Sista P, Miralles GD, et al. Enfuvirtide (T-20) and T-1249 resistance: observations from Phase II clinical trials of enfuvirtide in combination with oral antiretrovirals and a Phase I/II dose-ranging monotherapy trial of T-1249 [abstract no. 128]. Antiviral Ther 2002; 7 Suppl. 1: S140. Plus poster presented at the XI International HIV Drug Resistance Workshop; 2002 Jul 2–5; Seville
Furuta RA, Wild CT, Weng Y, et al. Capture of an early fusion-active conformation of HIV-1 gp41 [published erratum appears in Nat Struct Biol 1998 Jul; 5 (7): 612]. Nat Struct Biol 1998 Apr; 5(4): 276–9
Derdeyn CA, Decker JM, Sfakianos JN, et al. Sensitivity of human immunodeficiency virus type 1 to fusion inhibitors targeted to the gp41 first heptad repeat involves distinct regions of gp41 and is consistently modulated by gpl20 interactions with the coreceptor. J Virol 2001 Sep; 75(18): 8605–14
Greenberg ML, Melby T, Sista P, et al. Baseline and on-treatment susceptibility to enfuvirtide seen in TORO 1 and TORO 2 through 24 weeks [abstract no. 141 plus oral presentation]. 10th Conference on Retroviruses and Opportunistic Infections; 2003 Feb 10–14; Boston
Rimsky LT, Shugars DC, Matthews TJ. Determinants of human immunodeficiency virus type 1 resistance to gp41-derived inhibitory peptides. J Virol 1998 Feb; 72(2): 986–93
Sista P, Melby T, Greenberg ML, et al. Characterization of baseline and treatment-emergent resistance to T-20 (enfuvirtide) observed in phase II clinical trials: substitutions in gp41 amino acids 36–45 and enfuvirtide susceptibility of virus isolates [abstract no. 21]. Antiviral Ther 2002 Jun; 7(2): S16–S 17. Plus poster presented at the XI International HIV Drug Resistance Workshop; 2002 Jul 2–5; Seville
Mink M, Greenberg M, Mosier S, et al. Impact of HIV-1 gp41 amino acid substitutions (positions 36–45) on susceptibility to T-20 (enfuvirtide) in vitro: analysis of primary virus isolates recovered from patients during chronic enfuvirtide treatment and site-directed mutants in NL4-3 [abstract no. 22]. Antiviral Ther 2002 Jun; 7(2): S17–S 18. Plus poster presented at the XI International HIV Drug Resistance Workshop; 2002 Jul 2–5; Seville
Melby T, Sista P, Nelson E, et al. Virological characterization of patients through 48 weeks in T20-205 who acquired T-20 (enfuvirtide) resistance-associated mutations during prior short-term enfuvirtide monotherapy [abstract no. 70]. Antiviral Ther 2002; 7 Suppl. 1: S77. Plus poster presented at the XI International HIV Drug Resistance Workshop; 2002 Jul 2–5; Seville
Lu J, Sista P, Cammack N, et al. Fitness of HIV-1 clinical isolates resistant to T-20 (enfuvirtide) [abstract no. 67]. Antiviral Ther 2002; 7 Suppl. 1: S74. Plus poster presented at the XI International HIV Drug Resistance Workshop; 2002 Jul 2–5; Seville
Xu L, Hue S, Taylor S, et al. Minimal variation in T-20 binding domain of different HIV-1 subtypes from antiretroviral-naive and -experienced patients. AIDS 2002 Aug 16; 16(12): 1684–6
Sista P, Melby T, Dhingra U, et al. The fusion inhibitors T-20 and T-1249 demonstrate potent in vitro antiviral activity against clade B HIV-1 isolates resistant to reverse transcriptase and protease inhibitors and non-B clades [abstract no. 2]. Antiviral Ther 2001; 6 Suppl. 1: 3–4. Plus poster presented at the 5th International Workshop on HIV Drug Resistance and Treatment Strategies; 2001 Jun 4–8; Scottsdale, Arizona
Roman F, Gonzalez D, Lambert C, et al. Uncommon mutations at residue positions critical for enfuvirtide (T-20) resistance in enfuvirtide-naive patients infected with subtype B and non-B HIV-1 strains. J Acquir Immune Defic Syndr 2003 Jun 1; 33(2): 134–9
Hanna SL, Yang C, Owen SM, et al. Variability of critical epitopes within HIV-1 heptad repeat domains for selected entry inhibitors in HIV-infected population worldwide [published erratum appears in AIDS 2002 Sep 6; 16 (13):1847]. AIDS 2002 Aug 16; 16(12): 1603–6
Zollner B, Feucht HH, Schroter M, et al. Primary genotypic resistance of HIV-1 to the fusion inhibitor T-20 in long-term infected patients. AIDS 2001 May 4; 15(7): 935–6
Braun MC, Wang JM, Lahey E, et al. Activation of the formyl peptide receptor by the HIV-derived peptide T-20 suppresses interleukin-12 p70 production by human monocytes. Blood 2001 Jun 1; 97(11): 3531–6
Wheat LJ, Lalezari J, Kilby M, et al. A week-48 assessment of high strength T-20 formulations in multi-class experienced patients [abstract no. 417-W plus poster]. 9th Conference on Retroviruses and Opportunistic Infections; 2002 Feb 24–28; Seattle
Zhang X, Nieforth K, Lang JM, et al. Pharmacokinetics of plasma enfuvirtide after subcutaneous administration to patients with human immunodeficiency virus: inverse gaussian density absorption and 2-compartment disposition. Clin Pharmacol Ther 2002 Jul; 72(1): 10–9
Lalezari JP, Patel IH, Zhang X, et al. Influence of subcutaneous injection site on the steady-state pharmacokinetics of enfuvirtide (T-20) in HIV-1-infected patients. J Clin Virol 2003 Oct; 28(2): 217–22
Bellibas SE, Siddique Z, Dorr A, et al. Pharmacokinetics of enfuvirtide in pediatric HIV infected patients receiving combination therapy [abstract no. 841]. Antiviral Ther 2003; 8 Suppl. 1: S421. Plus poster presented at the 2nd IAS Conference on HIV Pathogenesis and Treatment; 2003 July 13–16; Paris
Boyd M, Ruxrungtham K, Zhang X, et al. Enfuvirtide-investigations in the drug interaction potential in HIV-infected patients [abstract no. 541 plus poster]. 10th Conference on Retroviruses and Opportunistic Infections; 2003 Feb 10–14; Boston
Zhang X, Patel IH, Lalezari JP, et al. Assessment of metabolic inhibition potential of enfuvirtide using a 5-drug cocktail in HIV-1 infected patients [poster P111-73]. American Society for Clinical Pharmacology and Therapeutics: 2003 Annual Meeting; 2003 Apr 2–5; Washington DC
Lalezari JP, Henry K, O’Hearn M, et al. Enfuvirtide, an HIV-1 fusion inhibitor, for drug-resistant HIV infection in North and South America. TORO 1 Study Group [published erratum appears in N Engl J Med 2003 Sep 11; 349(11): 1100]. N Engl J Med 2003 May 29; 348(22): 2175–85
Lazzarin A, Clotet B, Cooper D, et al. Efficacy of enfuvirtide in patients infected with drug-resistant HIV-1 in Europe and Australia. TORO 2 Study Group. N Engl J Med 2003 May 29; 348(22): 2186–95
Katlama C, Arasteh K, Clotet B, et al. Enfuvirtide TORO studies: 48 week results confirm 24 week findings [abstract no. LB2 plus poster]. 2nd IAS Conference on HIV Pathogenesis and Treatment: Late Breaker Abstracts; 2003 Jul 13–16; Paris, 280–1
Hornberger J, Green J. Clinical prognosis of enfuvirtide in combination with an optimized background regimen among categories of baseline CD4+ cell count and HIV antiretroviral resistance [abstract no. 478]. Antiviral Ther 2003; 8 Suppl. 1: S310. Plus poster presented at the 2nd IAS Conference on HIV Pathogenesis and Treatment; 2003 July 13–16; Paris
Clumeck N, Cohen CJ, Thompson M, et al. Impact of enfuvirtide on health-related quality of life at 48 weeks [poster no. 7.3/ 19]. 9th European AIDS Conference; 2003 Oct 25–29; Warsaw, Poland
Cohen C, Green J, Wintfeld N, et al. Patient acceptance with self-injection of enfuvirtide (ENF) for HIV over 48 weeks of treatment [poster no. 7.1/1]. 9th European AIDS conference; 2003 Oct 25–29; Warsaw, Poland
Green J, Salgo MP, Delehanty J. Patient survey on injection of T-20: ease of use and impact on activities [abstract no. TuPeB4480 plus poster]. 14th International Aids Conference; 2002 Jul 7–12; Barcelona, 387
Lalezari JP, DeJesus E, Northfelt DW, et al. A controlled phase II trial assessing three doses of enfuvirtide (T-20) in combination with abacavir, amprenavir, ritonavir and efavirenz in non-nucleoside reverse transcriptase inhibitor-naive HIV-infected adults. Antiviral Ther 2003 Aug; 8(4): 279–87
Church JA, Cunningham C, Hughes M, et al. Safety and antiretroviral activity of chronic subcutaneous administration of T-20 in human immunodeficiency virus 1-infected children. PACTG P1005 Study Team. Pediatr Infect Dis J 2002 Jul; 21(7): 653–9
Trimeris, Roche. New T-20 (enfuvirtide) research signals new hope for children and adolescents living with HIV T-20 paediatric dosing confirmed — plus early indications of good tolerability [online]. Available from URL: http://www.trimeris.com [Accessed 2003 Sep 3]
Eron J, Delfraissy J, Kuritzkes D, et al. Safety of enfuvirtide (ENF) through 48 weeks of therapy in the TORO trials [abstract no. H-836 plus poster]. 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC); 2003 Sep 14–17; Chicago, 312
Hornberger JC, Witek J, Kilby JM, et al. Clinical prognosis and cost-effectiveness of enfuvirtide (ENF) in the US [abstract no. H-837 plus poster]. 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC); 2003 Sep 14–17; Chicago, 312
Hornberger J, Youle M, Beck EJ, et al. Cost-effectiveness of enfuvirtide from UK health payer perspective [poster 19.5/1]. 9th European AIDS conference; 2003 Oct 25–29; Warsaw, Poland
Sax PE, Losina E, Weinstein MC, et al. Cost-effectiveness of a new fusion inhibitor (T-20) for patients who have failed antire-troviral therapy [abstract no. 577]. Antiviral Ther 2003; 8 Suppl. 1: S341
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dando, T.M., Perry, C.M. Enfuvirtide. Drugs 63, 2755–2766 (2003). https://doi.org/10.2165/00003495-200363240-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200363240-00005