Summary
Abstract
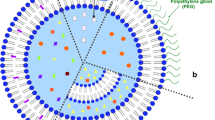
Polyethylene glycol (PEG)-liposomal doxorubicin is a formulation of the anthracycline doxorubicin in which the drug is encapsulated in PEG-coated liposomes. This alters the pharmacokinetic properties of doxorubicin, prolonging circulation time and enhancing localisation to tumours.
In a large randomised trial, intravenous PEG-liposomal doxorubicin was at least as effective as topotecan in patients with ovarian cancer refractory or sensitive to first-line platinum-based chemotherapy. Overall response rates of patients with ovarian cancer refractory to platinum- and paclitaxel-based chemotherapy who received the drug ranged from 18.3 to 27.6% in noncomparative clinical trials.
PEG-liposomal doxorubicin also has antitumour activity in patients with metastatic breast cancer pretreated with other chemotherapeutic agents. Overall response rates were similar in patients with pretreated metastatic breast cancer who had received PEG-liposomal doxorubicin or two comparator salvage chemotherapy regimens (vinorelbine or mitomycin C plus vinblastine) in an interim analysis of a large randomised study.
In patients with advanced AIDS-related Kaposi’s sarcoma, PEG-liposomal doxorubicin monotherapy produced overall response rates ranging from 46 to 77% in randomised trials. The drug was significantly more effective than bleomycin plus vincristine alone or in combination with standard doxorubicin, as measured by tumour response.
As a replacement for standard doxorubicin in commonly used combination therapies, PEG-liposomal doxorubicin has shown activity in multiple myeloma and aggressive non-Hodgkin’s lymphoma in small, preliminary trials.
The most common adverse events associated with PEG-liposomal doxorubicin are myelosuppression, palmar-plantar erythrodysaesthesia, stomatitis and nausea. These can be managed by delaying or reducing dosages. Although preliminary trials are promising, the relative cardiotoxicity of PEG-liposomal doxorubicin compared with the standard formulation has not been clearly established.
Conclusions: Monotherapy with PEG-liposomal doxorubicin is effective as a second-line chemotherapy in patients with platinum-refractory ovarian cancer and in patients with metastatic breast cancer. However, as with all chemotherapeutic agents, the benefits of treatment need to be weighed against the agent’s tolerability profile. Strong comparative data have helped to establish PEG-liposomal doxorubicin as the first-line treatment option in patients with advanced Kaposi’s sarcoma. Anticancer activity has also been observed in studies conducted in small numbers of patients with multiple myeloma or non-Hodgkin’s lymphoma receiving PEG-liposomal doxorubicin instead of standard doxorubicin in combination regimens, although further data are needed to confirm the clinical relevance of these findings.
Pharmacodynamic Properties
Polyethylene glycol (PEG)-liposomal doxorubicin consists of doxorubicin entrapped in PEG-coated liposomes. The antitumour activity of doxorubicin may arise mainly from interference with the topoisomerase II-DNA complex, resulting in fragmented DNA; other intracellular damage is caused by free radicals formed when the drug is metabolised. The latter mechanism is thought to be responsible not only for the antitumour activity of doxorubicin but also for adverse effects such as cardiotoxicity.
PEG-liposomal doxorubicin was more effective than standard doxorubicin against human ovarian carcinoma xenografts in mice. The liposomal formulation has also demonstrated activity in vitro against a range of different human tumour cell cultures including breast, ovarian and lymphoma tumour cell types. Higher concentrations of PEG-liposomal doxorubicin than standard doxorubicin were required to inhibit tumour cell proliferation for all cell lines. PEG-liposomal doxorubicin also strongly inhibits the in vitro growth of human Kaposi’s sarcoma spindle cells and Kaposi’s sarcoma lesions.
Pharmacokinetic Properties
PEG-liposomal doxorubicin has a different pharmacokinetic profile from that of standard doxorubicin, including a longer circulation time, slower clearance, smaller volume of distribution and a larger area under the plasma concentrationtime curve (AUC). In addition, PEG-liposomal doxorubicin delivers 5.2 to 11.4 times more doxorubicin to Kaposi’s sarcoma lesions than does the same dose of standard doxorubicin.
The plasma concentration profile of PEG-liposomal doxorubicin over a dose range of 10 to 20 mg/m2 was reported to be linear, while an increase in dose to 50 mg/m2 was associated with a nonlinear profile. After administration of PEG-liposomal doxorubicin 20 and 50 mg/m2, the AUC for doxorubicin was 564 and 902 mg · h/L, respectively, and the peak plasma doxorubicin concentration was 8.6 or 10.1 and 21.2 mg/L, respectively.
Limited data suggest that PEG-liposomal doxorubicin preferentially accumulates in tumour tissue because it has a prolonged circulation time. Once trapped in the tumour tissue interstitial fluid, the liposomes are thought to slowly release doxorubicin which can then enter and damage tumour cells. After PEG-liposomal doxorubicin administration, doxorubicin concentrations were about 10 to 20 times higher in Kaposi’s sarcoma lesions or bone metastases than in normal skin or tumour-free muscle, respectively.
Doxorubicin metabolites (e.g. doxorubicinol) were detected at low concentrations in urine, but were not detected or were detected in low concentrations in plasma after administration of PEG-liposomal doxorubicin. Clearance of single-dose PEG-liposomal doxorubicin 25 to 50 mg/m2 administered intravenously was independent of dose. Bile is likely to be the major route of doxorubicin excretion after administration of PEG-liposomal doxorubicin, based on results from animal studies.
The effect of hepatic dysfunction on PEG-liposomal doxorubicin pharmaco-kinetics has not yet been established. However, one study found no significant differences in volume of distribution and plasma clearance between patients with hepatocellular carcinoma receiving single-dose PEG-liposomal doxorubicin 20 or 30 mg/m2 intravenously and historical controls.
Clinical Efficacy
Ovarian cancer: In a large randomised study, PEG-liposomal doxorubicin 50 mg/m2 once every 4 weeks was at least as effective as topotecan 1.5 mg/m2 daily for 5 days every 3 weeks in 254 patients with ovarian cancer refractory to first-line platinum-based chemotherapy (overall response rates 12.3 vs 6.5%). The two treatments also produced similar rates of objective tumour response in 220 patients with disease sensitive to first-line platinum-based therapy (28.4 vs 28.8%) in the same study; however, patients receiving PEG-liposomal doxorubicin had significantly longer progression free- and overall survival times.
In subgroup analyses of noncomparative studies, PEG-liposomal doxorubicin 40 or 50 mg/m2 every 3 to 5 weeks produced overall response rates of 8.3 to 36.4% in 21 to 82 patients with ovarian cancer refractory or resistant to platinum-and paclitaxel-based chemotherapy.
In small, noncomparative studies, the drug has also shown efficacy in combination with topotecan, ifosfamide or gemcitabine in patients with recurrent or persistent ovarian cancer following platinum-based (and sometimes paclitaxel-based) therapy, and in combination with paclitaxel and carboplatin in chemotherapy-naïve patients with advanced ovarian cancer.
Advanced breast cancer: PEG-liposomal doxorubicin has antitumour activity in patients with metastatic disease that has already been treated with other chemotherapeutic agents. Overall response rates were similar in patients with pretreated metastatic breast cancer receiving PEG-liposomal doxorubicin 50 mg/m2 every 4 weeks or two comparator salvage chemotherapy regimens (vinorelbine or mitomycin C plus vinblastine) [13 vs 15%; no statistical comparison reported] in a randomised, large study (n = 301) published as an abstract. The combinations of PEG-liposomal doxorubicin and paclitaxel or vinorelbine in previously treated patients produced promising overall response rates of 48% and 18 to 36%, respectively, in small studies (n < 35).
Of 71 patients 28.2% experienced responses with PEG-liposomal doxorubicin in a multicentre study (39% of patients in this study were pretreated with chemotherapy).
Kaposi’s sarcoma: In randomised trials, PEG-liposomal doxorubicin monotherapy 20 mg/m2 every 2 to 3 weeks produced overall response rates of 46 to 77% in 126 to 258 patients.
The drug was significantly more effective than the commonly used regimen of bleomycin plus vincristine and standard doxorubicin (ABV). In two studies, PEG-liposomal doxorubicin 20 mg/m2 every 2 to 3 weeks also produced a greater response than bleomycin plus vincristine (BV). This response was only significantly greater in the larger of these studies. There was a trend towards longer mean duration of survival in patients receiving PEG-liposomal doxorubicin than those receiving BV (239 vs 160 days) and a similar median duration of survival to ABV (approximately 160 days).
Alone and in combination with BV (DBV), PEG-liposomal doxorubicin produced similar overall response rates in chemotherapy-naive patients; an interim analysis of this study determined that significantly fewer patients had died while receiving PEG-liposomal doxorubicin than while receiving DBV (18 vs 28%).
PEG-liposomal doxorubicin also has advantages over ABV in terms of improvements in health-related quality of life, and over both ABV and BV in reducing disfiguring characteristics and pain of indicator lesions.
Haematological malignancies: Substituting PEG-liposomal doxorubicin for standard doxorubicin in a vincristine, standard doxorubicin plus dexamethasone (VAD) regimen produced enough activity in small numbers of elderly patients with multiple myeloma to justify further studies of this regimen.
When substituted for standard doxorubicin in the commonly used standard doxorubicin plus cyclophosphamide, vincristine and methylprednisolone (CHOP) regimen, PEG-liposomal doxorubicin produced responses in all eight of the elderly patients with aggressive non-Hodgkin’s lymphoma receiving treatment. In other preliminary data, PEG-liposomal doxorubicin monotherapy produced overall response rates of 80 and 83% in patients with refractory non-Hodgkin’s lymphoma.
Tolerability
Preliminary results from a trial in 509 patients with metastatic breast cancer found a significantly lower risk of cardiac adverse events in patients receiving PEG-liposomal doxorubicin compared with standard doxorubicin.
Of 66 patients with solid tumours from pooled tolerability data who had received a cumulative dosage of >400 mg/m2 and had their left ventricular ejection fraction (LVEF) measured at baseline and follow-up, 12% experienced cardiotoxicity in the form of a decrease in LVEF of ≥20% from baseline or a change to <45%. Cardiotoxicity was also experienced by 1.4 to 3.4% of 45 to 132 patients with ovarian or breast cancer who received cumulative doses of PEG-liposomal doxorubicin ranging from 45 to 1301 mg/m2. 1.7 to 4.3% of patients with Kaposi’s sarcoma who received PEG-liposomal doxorubicin 20 mg/m2 every 2 or 3 weeks experienced cardiac-related adverse events thought to be possibly or probably related to PEG-liposomal doxorubicin. Significantly fewer cardiac histo-pathological changes were observed with a mean cumulative PEG-liposomal doxorubicin dose of 623 mg/m2 than in a historical patient group matched for cumulative dose who received standard doxorubicin.
The most common adverse events (grade I to IV severity) associated with PEG-liposomal doxorubicin as monotherapy in 512 patients with ovarian cancer were palmar-planter erythrodysaesthesia (PPE) [46.1%], stomatitis (38.9%) and nausea (38.1%) in pooled data. Reported haematological events included leucopenia (33.2%), anaemia (32.2%), neutropenia (31.6%), and thrombocytopenia (10.7%).
Haematological adverse events and alopecia were significantly less likely to occur with PEG-liposomal doxorubicin than with topotecan in patients with relapsed ovarian cancer in a randomised trial; however, PPE and stomatitis were significantly more common with PEG-liposomal doxorubicin than with topotecan.
The tolerability profile of PEG-liposomal doxorubicin in patients with Kaposi’s sarcoma differs from that in patients with solid tumours, possibly because of differences in dosages and concomitant therapies.
Myelosuppression is the dose-limiting adverse event experienced by patients with Kaposi’s sarcoma receiving PEG-liposomal doxorubicin; myelosuppression occurred in about 50% of patients in pooled tolerability data. Leucopenia was the most frequent event; neutropenia, thrombocytopenia and anaemia were also common.
PEG-liposomal doxorubicin appears to have similar overall incidences of adverse events as BV and ABV. However, PEG-liposomal doxorubicin was associated with less constipation and paraesthesia than BV and less nausea and/or vomiting, alopecia and peripheral neuropathy than ABV, but more leucopenia and opportunistic infections than BV and more mucositis and/or stomatitis than ABV.
Dosage and Administration
In the US, PEG-liposomal doxorubicin is indicated for the treatment of metastatic ovarian carcinoma that has progressed during paclitaxel- and platinum-based chemotherapy regimens or within 6 months of completing these treatments, and for the treatment of AIDS-related Kaposi’s sarcoma in patients with disease that has progressed during prior combination therapy, or in patients intolerant to such therapy. In Europe, the drug is indicated for the treatment of advanced ovarian cancer that has failed platinum-based chemotherapy regimens and the treatment of AIDS-related Kaposi’s sarcoma as either first- or second-line therapy. However, in these countries the drug is not to be used in the treatment of Kaposi’s sarcoma that may be treated effectively with local therapy or systemic interference. PEG-liposomal doxorubicin has not yet been approved for the treatment of metastatic breast cancer, multiple myeloma or non-Hodgkin’s lymphoma.
The recommended dosage of PEG-liposomal doxorubicin in patients with ovarian cancer is 50 mg/m2 administered intravenously once every 4 weeks. The drug should be administered by infusion at an initial rate of 1 mg/min, which can be increased if no infusion-related adverse events occur so that administration is completed in 1 hour. PEG-liposomal doxorubicin 20 mg/m2 as a 30-minute intravenous infusion once every 2 to 3 weeks is recommended for patients with Kaposi’s sarcoma. In both cancer types, treatment should continue for as long as patients respond satisfactorily and can tolerate therapy.
A delay or reduction of dosage is recommended if patients develop adverse events such as PPE, haematological adverse events or stomatitis. Precautions taken to avoid cardiotoxicity when administering standard doxorubicin should also be followed with PEG-liposomal doxorubicin. The cardiac function of patients receiving PEG-liposomal doxorubicin should be carefully monitored.














Similar content being viewed by others
Notes
Use of tradenames is for product identification only and does not imply endorsement.
References
Coukell A, Spencer CM. Polyethylene glycol-liposomal doxorubicin: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in the management of AIDS-related Kaposi’s sarcoma. Drugs 1997 Mar; 53(3): 520–38
Riggs CE, Bennett JP. Clinical pharmacology of individual antineoplastic agents. In: Moossa AR, Schimpff SC, Robson MC, editors. Comprehensive textbook of oncology. 2nd ed. v. 1. Baltimore, Maryland, USA: Williams & Wilkins, 1991: 537–65
Balmer C, Valley AW. Basic principles of cancer treatment and cancer chemotherapy. In: DiPiro JT, Talbert RL, Hayes PE, et al., editors. Pharmacotherapy: a pathophysiologic approach. 2nd ed. Norwalk, Connecticut, USA: Appleton & Lange, 1993: 1879–929
Waterhouse DN, Tardi PG, Mayer LD, et al. A comparison of liposomal formulations of doxorubicin with drug administered in free form: changing toxicity profiles. Drug Saf 2001; 24(12): 903–20
Olson RD, Mushlin PS. Doxorubicin cardiotoxicity: analysis of prevailing hypotheses. FASEB J 1990 Oct; 4(13): 3076–86
Speyer J, Wasserheit C. Strategies for reduction of anthracycline cardiac toxicity. Semin Oncol 1998; 25(5): 525–37
Green M. Anthracycline cardiotoxicity, no longer an issue? Ann Oncol 1998; 9: 691–3
Gabizon A, Martin F. Polyethylene glycol-coated (pegylated) liposomal doxorubicin. Rationale for use in solid tumours. Drugs 1997; 54 Suppl. 4: 15–21
Lasic DD. Doxorubicin in sterically stabilized liposomes. Nature 1996 Apr 11; 380: 561–2
Lasic DD, Martin FJ, Gabizon A, et al. Sterically stabilized liposomes: a hypothesis on the molecular origin of the extended circulation times. Biochim Biophys Acta 1991; 1070: 187–92
Meriwether WD, Bachur NR. Inhibition of DNA and RNA metabolism by daunorubicin and adriamycin in L1210 mouse leukemia. Cancer Res 1972 Jun; 32(6): 1137–42
Reinert KE. Anthracycline-binding induced DNA stiffening, bending and elongation; stereochemical implications from viscometric investigations. Nucleic Acids Res 1983 May 25; 11(10): 3411–30
Hortobágyi GN. Anthracyclines in the treatment of cancer. Drugs 1997; 54 Suppl. 4: 1–7
Beyer U, Rothen-Rutishauser B, Unger C, et al. Differences in the intracellular distribution of acid-sensitive doxorubicin-protein conjugates in comparison to free and liposomal formulated doxorubicin as shown by confocal microscopy. Pharm Res 2001; 18(1): 29–38
Stürzl M, Zietz C, Eisenburg B, et al. Liposomal doxorubicin in the treatment of AIDS-associated Kaposi’s sarcoma: clinical, histological and cell biological evaluation. Res Virol 1994 May–Aug; 145(3-4): 261–9
Vaage J, Donovan D, Mayhew E, et al. Therapy of human ovarian carcinoma xenografts using doxorubicin encapsulated in sterically stabilized liposomes. Cancer 1993 Dec 15; 72: 3671–5
Wiles ME, Bell C, Landfair D, et al. Anthracycline efficacy in vitro: cytotoxicity of liposomal/nonliposomal daunorubicin and doxorubicin for multiple tumor cell types [in ENGLISH]. Drug Delivery: Journal of Delivery and Targeting of Therapeutic Agents 1997; 4(4): 255–62
Pratt G, Wiles ME, Rawstron AC, et al. Liposomal daunorubicin: in vitro and in vivo efficacy in multiple myeloma. Hematol Oncol 1998 Jun; 16: 47–55
Gabizon A, Chemla M, Tzemach D, et al. Liposome longevity and stability in circulation: effects on the in vivo delivery to tumors and therapeutic efficacy of encapsulated anthracyclines. J Drug Target 1996; 3: 391–8
Cabanes A, Tzemach D, Goren D, et al. Comparative study of the antitumor activity of free doxorubicin and polyethylene glycol-coated liposomal doxorubicin in a mouse lymphoma model. Clin Cancer Res 1998 Feb; 4: 499–505
Vaage J, Donovan D, Mayhew E, et al. Therapy of mouse mammary carcinomas with vincristine and doxorubicin encapsulated in sterically stabilized liposomes. Int J Cancer 1993; 54: 959–64
Vaage J, Mayhew E, Lasic D, et al. Therapy of primary and metastatic mouse mammary carcinomas with doxorubicin encapsulated in long circulating liposomes. Int J Cancer 1992; 51: 942–8
Vaage J, Donovan D, Loftus T, et al. Chemoprevention and therapy of mouse mammary carcinomas with doxorubicin encapsulated in sterically stabilized liposomes. Cancer 1994 May 1; 73: 2366–71
Vaage J, Donovan D, Loftus T, et al. Prevention of metastasis from mouse mammary carcinomas with liposomes carrying doxorubicin. Br J Cancer 1995; 72(5): 1074–5
Vaage J, Donovan D, Loftus T, et al. Prophylaxis and therapy of mouse mammary carcinomas with doxorubicin and vincristine encapsulated in sterically stabilised liposomes. Eur J Cancer 1995; 31A(3): 367–72
Lyass O, Uziely B, Ben-Yosef R, et al. Correlation of toxicity with pharmacokinetics of pegylated liposomal doxorubicin (Doxil) in metastatic breast carcinoma. Cancer 2000; 89(5): 1037–47
Amantea MA, Gabizon A. Pharmacokinetics (PKs) of Caelyx®/Doxil® (Stealth® liposomal doxorubicin) in patients with either breast or prostate cancer [abstract]. Ann Oncol 1998; 9 Suppl. 2: 171
Gabizon A, Uziely B, Lotem M, et al. Doxil® in patients with pretreated metastatic breast cancer (MBC): a dose-schedule finding study with pharmacokinetics [abstract]. 33rd Proc Am Soc Clin Oncol 1997 May 17; 16: 147
Symon Z, Peyser A, Tzemach D, et al. Selective delivery of doxorubicin to patients with breast carcinoma metastases by stealth liposomes. Cancer 1999 Jul 1; 86: 72–8
Czejka M, Braunsdorfer M, Strauch S, et al. Pegylated liposomal doxorubicin (Caelyx®), administered intravenously at conventional dosages, penetrates into the brain [abstract no. 448]. Clin Cancer Res 1999 Nov; 5 Suppl.: 3819s
Stewart S, Harrington KJ. The biodistribution and pharmacokinetics of stealth liposomes in patients with solid tumors. Oncology USA 1997; 11 (10 Suppl. 11): 33–7
Amantea MA, Forrest A, Northfelt DW, et al. Population pharmacokinetics and pharmacodynamics of pegylated-liposomal doxorubicin in patients with AIDS-related Kaposi’s sarcoma. Clin Pharmacol Ther 1997 Mar; 61: 301–11
Weger M, Czejka M, Linkesch W, et al. A phase I pharmaco-kinetic study of Doxil® (liposomal doxorubicin in combination with vinorelbine, cyclophosphamide, and prednisone in NHL patients [abstract no. 894]. 35th ASCO; 1999 May 15–18; Atlanta, Georgia: 232
Gautier M, Petros W, Peterson B, et al. Doxil, vincristine and dexamethasone: a new regimen for elderly patients with multiple myeloma [abstract no. 101]. 35th ASCO; 1999 May 15–18; Atlanta, Georgia: 28a
Venook AP, Amantea M, Bonnern E, et al. Pegylated liposome-encapsulated doxorubicin (Doxil®) in patients with hepa-tocellular carcinoma [abstract no. 1494]. 32nd ASCO; 1996 May 18–21; Philadelphia, PA: 473
Schering-Plough Ltd. Caelyx prescribing information. Bruxelles, Belgium, Jul 2001
Gabizon A, Catane R, Uziely B, et al. Prolonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulated in polyethylene-glycol coated liposomes. Cancer Res 1994 Feb 15; 54: 987–92
Northfelt DW, Martin FJ, Working P, et al. Doxorubicin encapsulated in liposomes containing surface-bound polyethylene glycol: pharmacokinetics, tumor localization, and safety in patients with AIDS-related Kaposi’s sarcoma. J Clin Pharmacol 1996 Jan; 36(1): 55–63
Gabizon A, Huang A, Martin F, et al. Doxorubicin encapsulated in polyethylene glycol-coated liposomes: initial clinicalpharmacokinetic studies in solid tumors. In: Lasic D, Martin F, editors. Stealth liposomes. Boca Raton, Florida, USA: CRC Press, 1995:245–55
Guyton AC. Textbook of medical physiology. 6thed. Philadelphia: W.B. Saunders Company, 1981
Allen TM. Liposomes: opportunities in drug delivery. Drugs 1997; 54 Suppl. 4: 8–14
Brown JM, Giaccia AJ. The unique physiology of solid tumors: Opportunities (and problems) for cancer therapy. Cancer Res 1998; 58(7): 1408–16
ALZA Pharmaceuticals Inc. Doxil® prescribing information. Mountain View, California, USA, Jul 2000
Harrington KJ, Gooden CSR, Mohammedtaghi S, et al. Biodistribution and pharmacokinetics of In-111 labeled Stealth® liposomes in patients with solid tumours [abstract no. 1511]. 32nd ASCO; 1996 May 18–21; Philadelphia, PA: 477
Gabizon AA. Liposomal anthracyclines. New Drug Therapy 1994; 8(2): 431–50
Northfelt DW. Stealth® liposomal doxorubicin (SLD) delivers more doxorubicin (DOX) to AIDS-Kaposi’s sarcoma (AIDS-KS) lesions than to normal skin [abstract no. 5]. Proc Am Soc Clin Oncol 1994 Mar; 13: 51
Working PK, Newman MS, Huang SK, et al. Pharmacokinetics, biodistribution and therapeutic efficacy of doxorubicin encapsulated in Stealth® liposomes (Doxil®). J Liposome Res 1994; 4(1): 667–87
Working PK, Dayan AD. Pharmacological-toxicological expert report. Caelyx™. (Stealth® liposomal doxorubicin HCl). Hum Exp Toxicol 1996; 15(9): 752–85
Huang SK, Lee K-D, Hong K, et al. Microscopic localization of sterically stabilized liposomes in colon carcinoma-bearing mice. Cancer Res 1992 Oct 1; 52: 5135–43
Harrington K, Stewart S, Harrison D, et al. Phase II pilot study of Caelyx (doxorubicin HC1, pegylated liposomal) in patients with inoperable head and neck squamous cell cancer [abstract]. Br J Cancer 1998; 78 Suppl. 2: 34
Koukourakis MI, Koukouraki S, Giatromanolaki A, et al. Liposomal doxorubicin and conventionally fractionated radiotherapy in the treatment of locally advanced non-small-cell lung cancer and head and neck cancer. J Clin Oncol 1999 Nov; 17: 3512–21
Caponigro F, Cornelia P, Budillon A, et al. Phase I study of Caelyx (doxorubicin HCL, pegylated liposomal) in recurrent or metastatic head and neck cancer. Ann Oncol 2000; 11: 339–42
Stewart JS, Lewanski C, Harrington K, et al. Comparison of pegylated liposomal doxorubicin (PLD) and pegylated liposomal cisplatinum (PLC) in patients with advanced head and neck squamous cell carcinoma (HNSCC) [abstract no. 1672]. 36th ASCO; 2000 May 20–23; New Orleans, LA: 423
Halm U, Etzrodt G, Schiefke I, et al. A phase II study of pegylated liposomal doxorubicin for treatment of advanced hepatocellular carcinoma. Ann Oncol 2000 Jan; 11: 113–4
Hubert A, Lyass O, Pode D, et al. Doxil (Caelyx): an exploratory study with pharmacokinetics in patients with hormone-refractory prostate cancer. Anticancer Drugs 2000; 11: 123–7
McMenemin R, Macdonald G, Moffat L, et al. A phase II study of Caelyx™ (liposomal doxorubicin) in metastatic carcinoma of the prostate: tolerability and efficacy modification by liposomal encapsulation. Invest New Drugs 2002; 20(3): 331–7
Toma S, Tucci A, Villani G, et al. Liposomal doxorubicin (Caelyx) in advanced pretreated soft tissue sarcomas: a phase II study of the Italian Sarcoma Group (ISG). Anticancer Res 2000; 20: 485–92
Dietrich J, Fabel-Schulte K, Hau P, et al. Liposomal doxorubicin (Caelyx) in the treatment of recurrent high-grade glioma — a phase II/III study [abstract no. 661]. Proc Am Soc Clin Oncol 2000 May 20; 19: 170
Dietrich J, Hau P, Fabel K, et al. Phase II clinical trial in high-grade-glioma: stabilization of disease in patients treated with liposomal doxorubicin [abstract no. 262]. Proc Am Soc Clin Oncol 2001 May 12; 20 Pt 1: 66
Oh Y, Perez-Soler R, Fossella FV, et al. Phase II study of intravenous Doxil®in malignant pleural mesothelioma. Invest New Drugs 2000; 18: 243–5
Baas P, van Meerbeeck J, Groen H, et al. Caelyx™ in malignant mesothelioma: a phase II EORTC study. Ann Oncol 2000; 11: 697–700
Lorigan PC, Crosby T, Coleman RE. Current drug treatment guidelines for epithelial ovarian cancer. Drugs 1996 Apr; 51(4): 571–84
Wiseman LR, Spencer CM. Paclitaxel: an update of its use in the treatment of metastatic breast cancer and ovarian and other gynaecological cancers. Drugs Aging 1998 Apr; 12(4): 305–34
Gordon AN, Fleagle JT, Guthrie D, et al. Recurrent epithelial ovarian carcinoma: a randomized phase III study of pegylated liposomal doxorubicin versus topotecan. J Clin Oncol 2001 Jul 15; 19(14): 3312–22
Gibbs DD, Pyle L, Allen M, et al. A phase I dose-finding study of a combination of pegylated liposomal doxorubicin (Doxil), carboplatin and paclitaxel in ovarian cancer. Br J Cancer 2002; 86(9): 1379–84
Green S, Weiss GR. Southwest Oncology Group standard response criteria, endpoint definitions and toxicity criteria. Invest New Drugs 1992; 10: 239–53
Muggia FM, Hainsworth JD, Jeffers S, et al. Phase II study of liposomal doxorubicin in refractory ovarian cancer: antitumor activity and toxicity modification by liposomal encapsulation. J Clin Oncol 1997 Mar; 15(3): 987–93
Safra T, Groshen S, Jeffers S, et al. Treatment of patients with ovarian carcinoma with pegylated liposomal doxorubicin: analysis of toxicities and predictors of outcome. Cancer 2001; 91: 90–100
Gordon AN, Granai CO, Rose PG, et al. Phase II study of liposomal doxorubicin in platinum- and paclitaxel-refractory epithelial ovarian cancer. J Clin Oncol 2000 Sep; 18(17): 3093–100
Rose PG, Maxson JH, Fusco N, et al. Liposomal doxorubicin in ovarian, peritoneal, and tubal carcinoma: a retrospective comparative study of single-agent dosages. Gynecol Oncol 2001; 82: 323–8
Rustin GJS, Nelstrop AE, McClean P, et al. Defining response of ovarian carcinoma to initial chemotherapy according to serum CA 125. J Clin Oncol 1996 May; 14(5): 1545–51
Campos SM, Penson RT, Mays AR, et al. The clinical utility of liposomal doxorubicin in recurrent ovarian cancer. Gynecol Oncol 2001; 81: 206–12
Israel VP, Garcia AA, Roman L, et al. Phase II study of liposomal doxorubicin in advanced gynecologic cancers. Gynecol Oncol 2000; 78: 143–7
Arcuri C, Sorio LR, Tognon G, et al. Efficacy and toxicity of Doxil/Caelyx (D) in patients with recurrent epithelial ovarian cancer (OC) [abstract no. C13]. Ann Oncol 2001; 12 Suppl. 4: 34
National Institute for Clinical Excellence. Guidance on the use of pegylated liposomal doxorubicin hydrochloride (PLDH) for the treatment of advanced ovarian cancer. Technology Appraisal Guidance — No. 45, Jul 2002. Availalbe from URL: http://www.nice.org.uk.[Accessed 26 Jul 2002]
Voest EE, Verhaar-Langereis MJ. A phase II study of the combination doxil and topotecan in platinum-resistant ovarian cancer [abstractno. 2479]. 37th ASCO; 2001 May 12–15; San Francisco, CA: 182
Geertsen PF, Str0yer I, Herrstedt J, et al. Phase I study of topotecan (T) and pegylated liposomal doxorubicin (Caelyx) in patients (pts) with progressive ovarian cancer within 12 months after first-line platinum-paclitaxel containing chemotherapy [abstract no. 2522]. 37th ASCO; 2001 May 12–15; San Francisco, CA: 193b
Mirchandani D, Höchster H, Hamilton A, et al. Efficacy of Doxil and topotecan in platinum-pretreated epithelial ovarian cancer in a phase I study [abstract no. 138]. Gynecol Oncol 2001 Feb; 80(2): 315
Bourgeois H. Pegylated liposomal doxorubicin (Caelyx) and ifosfamide (IFO) in recurrent ovarian cancer (ROC): a phase I/II GINECO study [abstract no. 1577]. 36th ASCO; 2000 May 20–23; New Orleans, LA: 398a
Tobias DH, Runowicz J, Mandeli J, et al. A phase I trial of gemcitabine and doxil for recurrent epithelial ovarian cancer [abstract no. 1551]. 36th ASCO; 2000 May 20–23; New Orleans, LA: 392a
Alberg AJ, Lam AP, Helzlsouer KJ. Epidemiology, prevention and early detection of breast cancer. Curr Opin Oncol 1999; 11: 435–41
Rubens RD. Key issues in the treatment of advanced breast cancer: expectations and outcomes. Pharmacoeconomics 1996; 9 Suppl. 2: 1–7
Keller AM, Mennel RG, Nabholtz J, et al. Phase III trial of pegylated liposomal doxorubicin (Caelyx/Doxil) for the treatment of patients with advanced breast cancer who have failed a prior taxane-containing chemotherapy regimen [abstract no. 115 and poster]. 37th ASCO; 2001 May 12–15; San Francisco, CA: 30a
Smith FP, Barr F, Hendricks C, et al. Phase II study of Doxil® (pegylated liposomal doxorubicin) in doxorubicin-resistant, metastatic breast cancer [abstract 524]. 35th ASCO; 1999 May 15–18; Atlanta, Georgia: 137
Modiano M, Taylor C, Sharpington T, et al. Phase I study of Doxil®(pegylated liposomal doxorubicin) plus escalating doses of Taxol®in the treatment of patients with advanced breast or gynecologic malignancies [abstract]. 35th ASCO; 1999 May 15–18; Atlanta, Georgia: 220
Schwonzen M, Kurbacher CM, Mailmann P. Liposomal doxorubicin and weekly paclitaxel in the treatment of metastatic breast cancer. Anticancer Drugs 2000; 11: 681–5
Burstein HJ, Ramirez MJ, Petros WP, et al. Phase I study of Doxil and vinorelbine in metastatic breast cancer. Ann Oncol 1999 Sep; 10: 1113–6
Martin M, Casado A, Garcia-Carbonero I, et al. Phase II study of pegilated liposomal doxorubicin plus vinorelbine in metastatic breast cancer patients with prior anthracycline treatment [abstract no. 1965]. 38th ASCO; 2002 May 18–21; Orlando, Florida
Rimassa L, Salvini P, Carnaghi C, et al. Unexpected low efficacy of caelyx and vinorelbine in metastatic breast cancer (MBC) [abstractno. 446]. 36th ASCO; 2000 May 20–23; New Orleans, LA: 115
Rivera E, Valero V, Syrewicz L, et al. Phase I study of stealth liposomal doxorubicin in combination with gemcitabine in the treatment of patients with metastatic breast cancer. J Clin Oncol 2001 Mar 15; 19(6): 1716–22
Park JW, Stauffer P, Diederich C, et al. Hyperthermia (HT) + Doxil significantly enhances drug delivery and efficacy in metastatic breast cancer of the chest wall (CW): a phase I/II study [abstract no. 184]. 37th ASCO; 2001 May 1–15; San Francisco, CA: 47a
Ranson MR, Carmichael J, O’Byrne K, et al. Treatment of advanced breast cancer with sterically stabilized liposomal doxorubicin: results of a multicenter phase II trial [see comments]. J Clin Oncol 1997 Oct; 15: 3185–91
Sparano JA, Malik U, Rajdev L, et al. Phase I trial of pegylated liposomal doxorubicin and docetaxel in advanced breast cancer. J Clin Oncol 2001 Jun 15; 19(12): 3117–25
Wigler N, Inbar M, O’Brien M, et al. Reduced cardiac toxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin (Caelyx/Doxil) vs doxorubicin for first-line treatment of metastatic breast cancer [abstract no. 177]. 38th Annual Meeting of the American Society of Clinical Oncology; 2002 May 18–21; Orlando, Florida
Miller AB, Hoogstraten B, Staquet M, et al. Reporting results of cancer treatment. Cancer 1981 Jan; 47: 207–14
Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982; 5: 649–55
Jahanzeb M, Frankel C, Elkersh M, et al. Rationale for trials studying pegylated liposomal doxorubicin in metastatic breast cancer. Oncology USA 1997; 11 (10 Suppl. 11): 45–53
National Cancer Institute Cancemet. Breast cancer (PDQ®) treatment — health professionals [online]. Available from URL: http://cancernet.nci.nih.gov[Accessed 2002 Jun 21]
Gogas H, Papadimitriou C, Kalofonos HR, et al. Combination of liposomal doxorubicin (Caelyx) and paclitaxel in locally advanced breast cancer. A phase II study of the Hellenic Cooperative Oncology Group (HeCOG) [abstract no. 2007]. 38th ASCO; 2002 May 18–21; Orlando, Florida
Vorobiof DA, Rapoport BL, McMichael GB, et al. Paclitaxel (P) and pegylated liposomal doxorubicin (PLD) as first line therapy forpatients (pts) with metastatic breast cancer (MBC) [abstract no. 2062]. 38th ASCO; 2002 May 18–21; Orlando, Florida
Betageri GV, Dipali SR. Preparation and in vitro dissolution profiles of tolazamide-polyethylene glycol solid dispersions. Drug Dev Ind Pharm 1995; 21(11): 1347–52
Mitsuyasu RT. AIDS-relatedKaposi’s sarcoma: current treatment options, future trends. Oncology 2000 Jun; 14(6): 867–78
Mitsuyasu R, yon Roenn J, Krown R, et al. Comparison study of liposomal doxorubicin (DOX) alone or with bleomycin and vincristine (DBV) for treatment of advanced AIDS-associated Kaposi’s sarcoma (AIDS-KS): AIDS clinical trial group (ACTG) protocol 286 [abstract no. 191]. 33rd ASCO; 1997 May 17–20; Denver, CO: 55a
Northfelt DW, Dezube BJ, Thommes JA, et al. Pegylated-liposomal doxorubicin versus doxorubicin, bleomycin, and vincristine in the treatment of AIDS-related Kaposi’s sarcoma: results of a randomized phase III clinical trial. J Clin Oncol 1998 Jul; 16: 2445–51
Rizzardini G, Pastecchia C, Vigevani GM, et al. Stealth liposomal doxorubicin or bleomycin/vincristine for the treatment of AIDS-related Kaposi’s sarcoma [abstractno. 17]. J Acquir Immune Defic Syndrom Hum Retrovirol 1997 Apr 1; 14(4): A20
Stewart S, Jablonowski H, Goebel FD, et al. Randomized comparative trial of pegylated liposomal doxorubicin versus bleomycin and vincristine in the treatment of AIDS-related Kaposi’s sarcoma. International Pegylated Liposomal Doxorubicin Study Group. J Clin Oncol 1998 Feb; 16: 683–91
Northfelt DW, Dezube BJ, Thommes JA, et al. Efficacy of pegylated-liposomal doxorubicin in the treatment of AIDS-related Kaposi’ s sarcoma after failure of standard chemotherapy. J Clin Oncol 1997 Feb; 15: 653–9
Hengge UR, Esser S, Rudel HP, et al. Long-term chemotherapy of HIV-associated Kaposi’s sarcoma with liposomal doxorubicin. Eur J Cancer 2001; 37: 878–83
Krown S, Metroka C, Wernz JC. Kaposi’s sarcoma in the Aquired Immune Deficiency Syndrome: a proposal for uniform evaluation, reponse, and staging criteria. J Clin Oncol 1989 Sep;7(9): 1201–7
Osoba D, Northfelt DW, Budd DW, et al. Effect of treatment on health-related quality of life in acquired immunodeficiency syndrome (AIDS)-related Kaposi’s sarcoma: a randomized trial of pegylated-liposomal doxorubicin versus doxorubicin, bleomycin, and vincristine. Cancer Invest 2001; 19(6): 573–80
Wu AW, Rubin HR, Mathews WC, et al. A health status questionnaire using 30 items from the medical outcomes study: preliminary validation in persons with early HIV infection. Med Care 1991 Aug; 29(8): 786–98
San Miguel JF, Creixenti JB, Garcia-Sanz R. Treatment of multiple myeloma. Haematologica 1999; 84: 36–58
National Cancer Institute Cancernet. Multiple myeloma and other plasma cell neoplasms (PDQ®) treatment — health professionals. Available from http://cancernet.nci.nih.gov [date last modified: Oct 2001]
Tsiara SN, Kapsali E, Christou L, et al. Administration of a modified chemotherapeutic regimen containing vincristine, liposomal doxorubicin and dexamethasone to multiple myeloma patients: preliminary data. Eur J Haematol 2000; 65: 118–22
Hussein MA, Wood L, McLain D, et al. Phase II study of Doxil® (DO), vincristine (V) and Decadron®(D) [DVD]: in newly diagnosed multiple myeloma (MM) patients (pts) [abstract]. Blood 1998 Nov 15 Suppl. 1 (Pt 2): 278–9
Hussein M, Karam M, Wood L, et al. Doxil (D), vincristine (V), and decadron (DX): a tolerable salvage regimen for relapsed refractory patients (pts) with multiple myeloma (MM) [abstract no. 4615]. Blood 1999 Nov 15; 94(10) Suppl. 1 (Pt 2): 311b
National Cancer Institute CancerNet. Adult non-Hodgkin’s lymphoma (PDQ®) treatment-health professionals [online]. Available from URL: http://cancernet.nci.nih.gov [Accessed 2002 Jun 21]
MooreJr DF, Cabanillas F. Overview of prognostic factors in non-Hodgkin’s lymphoma. Oncology 1998 Oct; 12 (10 Suppl. 8): 17–24
Dranitsaris G. Treatment of non-Hodgkin’s lymphoma. Anti-cancer Drugs 1998; 9: 879–88
Niitsu N. Non-Hodgkin’s lymphoma in the elderly: a guide to drug treatment. Drugs Aging 1999 Jun; 14(6): 447–57
Tsavaris N, Katsikas M, Kosmas C, et al. Substitution of doxorubicin by pegylated liposomal doxorubicin (Caelyx) in the CHOP chemotherapy regimen in elderly (>75 years) patients with aggressive non-Hodgkin’s lymphoma (NHL): preliminary experience [abstract]. 9th Anticancer; 1999 Feb 2–5; Paris: 251
Tulpule A, Justice g, Espina BM, et al. Pegylated liposomal doxorubicin (Doxil®) is active in the treatment of relapsed/refractory lymphomas [abstract]. Blood 1998 Nov 15 Suppl. 1 (Pt 2):241
Wollina U, Graefe T, Karte K. Treatment of relapsing or recalcitrant cutaneous T-cell lymphoma with pegylated liposomal doxorubicin. J Am Acad Dermatol 2000 Jan; 42 (Pt 1): 40–6
Pharmacia & Upjohn. Adriamycin prescribing information. Kalamazoo, Michigan, USA, 1999
Safra T, Muggia F, Jeffers S, et al. Pegylated liposomal doxorubicin (Doxil): reduced clinical cardiotoxicity in patients reaching or exceeding cumulative doses of 500 mg/m2. Ann Oncol 2000; 11: 1029–33
Berry G, Billingham M, Alderman E, et al. The use of cardiac biopsy to demonstrate reduced cardiotoxicity in AIDS Kaposi’s sarcoma patients treated with pegylated liposomal doxorubicin. Ann Oncol 1998 Jul; 9: 711–6
Alberts DS, Garcia DJ. Safety aspects of pegylated liposomal doxorubicin in patients with cancer. Drugs 1997; 54 Suppl. 4: 30–5
Kollmannsberger C, Mayer F, Harstrick A, et al. Reduction of skin toxicity of pegylated liposomal doxorubicin (PLD) by concomitant administration of dexamethasone and pyridoxin in patients (pts) with anthracyclin-sensitive malignancies — a phase I/II trial [abstract no. 623P]. Ann Oncol 2000 Oct 13; 11 Suppl. 4: 136
Lopez AM, Wallace L, Dorr RT, et al. Topical DMSO treatment for pegylated liposomal doxorubicin-induced palmar-plantar erythrodysesthesia. Cancer Chemother Pharmacol 1999; 44: 303–6
Ozols RF. Update of the NCCN Ovarian Cancer Practice Guidelines. Oncology 1997 Nov; 11 (11A) NCCN Proceedings: 95–105
McGuire WP, Hoskins WJ, Brady MF, et al. Cyclophosph-amide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med 1996 Jan 4; 334: 1–6
Bookman MA, McGuire WP, Kilpatrick D, et al. Carboplatin and paclitaxel in ovarian carcinoma: a phase I study of the Gynecologic Oncology Group. J Clin Oncol 1996 Jun; 14: 1895–902
Gibbs DD, Gore ME. Pursuit of optimum outcomes in ovarian cancer: methodological approaches to therapy. Drugs 2001; 61(8): 1103–20
Gibbs D, Pyle L, Allen M, et al. A phase I, dose-finding study of carboplatin (C), paclitaxel (P) and liposomal doxorubicin (LD) in advanced epithelial ovarian carcinoma (EOC) [abstract no. 1539]. Proc Am Soc Clin Oncol 2000 May 20; 19: 389a
Hortobagyi GN. Treatment of breast cancer. N Engl J Med 1998 Oct 1; 339(14): 974–84
Fornier M, Munster P, Scidman AD. Update on the management of advanced breast cancer. Oncology 1999 May; 13(5): 647–58
National Comprehensive Cancer Network. NCCN practice guidelines for breast cancer: version 2000. Oncology 2000 Nov; 14(11A): 33–49
Lebwohl DE, Canetta R. New developments in chemotherapy of advanced breast cancer. Ann Oncol 1999; 10 Suppl. 6: S139–46
Aapro MS. Combining new agents with anthracyclines in metastatic breast cancer: an overview of recent findings. Semin Oncol 1999 Feb; 26 (1) Suppl. 3: 17–21
Figgitt DP, Wiseman LR. Docetaxel: an update of its use in advanced breast cancer. Drugs 2000 Mar; 59(3): 621–51
Carlson RW, Anderson BO, Bensinger W, et al. Breast cancer practice guidelines [online]. Available from: URL: http://www.nccn.org [Accessed 2002 Mar 20]
Nasti G, Errante D, Santarossa S, et al. A risk and benefit assessment of treatment for AIDS-related Kaposi’s sarcoma. Drug Saf 1999 May; 20(5): 403–25
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: D.S. Alberts, Arizona Cancer Center, Tucson, Arizona, USA; P.F. Conte, Azienda Ospedaliera Pisana, Pisa, Italy; M. De Lena, Medical Oncology Division, Oncology Institute, Bari, Italy; A.A. Gabizon, Department of Oncology, Hadassah Hebrew University Medical Center, Jerusalem, Israel; D. Goldstein, Institute of Oncology, University of New South Wales, Randwick, New South Wales, Australia; F.M. Muggia, Medical Oncology and Clinical Investigators, Kaplan Cancer Center, New York, New York, USA.
Data Selection
Sources: Medical literature published in any language since 1983 on pegylated-liposomal-doxorubicin, identified using Medline and EMBASE, supplemented by AdisBase (a proprietary database of Adis International Limited). Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: Medline search terms were ‘pegylated-liposomal-doxorubicin’ or ‘doxil’ or ‘dox SL’. EMBASE search terms were ‘pegylated-liposomal-doxorubicin’ or ‘DOX-SL’ or ‘doxil’. AdisBase search terms were ‘pegylated-liposomal-doxorubicin’ or ‘doxil’ or ‘dox-SL’. Searches were last updated 20 August 2002.
Selection: Studies in patients with ovarian cancer, breast cancer, multiple myeloma, non-Hodgkin’s lymphoma or Kaposi’s sarcoma who received polyethylene glycol-liposomal doxorubicin. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Polyethylene glycol-liposomal doxorubicin, pegylated-liposomal doxorubicin, ovarian cancer, breast cancer, multiple myeloma, non-Hodgkin’s lymphoma, Kaposi’s sarcoma, pharmacodynamics, pharmacokinetics, therapeutic use, adverse events.
Rights and permissions
About this article
Cite this article
Sharpe, M., Easthope, S.E., Keating, G.M. et al. Polyethylene Glycol-Liposomal Doxorubicin. Drugs 62, 2089–2126 (2002). https://doi.org/10.2165/00003495-200262140-00012
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200262140-00012