Abstract
-
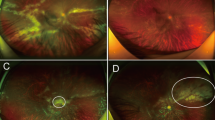
▴ Valganciclovir is a prodrug of ganciclovir which has been developed for the treatment of cytomegalovirus (CMV) retinitis in patients with AIDS.
-
▴ Oral valganciclovir is rapidly absorbed and hydrolysed to ganciclovir. The oral bioavailability of ganciclovir after oral valganciclovir administration is high. Oral valganciclovir 900mg provides a daily exposure of ganciclovir comparable to that of intravenous ganciclovir 5 mg/kg.
-
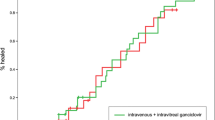
▴ A single, randomised, nonblind study indicated that oral valganciclovir (900mg twice daily for 3 weeks then 900mg once daily) and intravenous ganciclovir (5 mg/kg twice daily for 3 weeks then 5 mg/kg once daily) were equally effective in the treatment of newly diagnosed CMV retinitis in 160 patients with AIDS.
-
▴ Valganciclovir appears to have a similar tolerability profile to intravenous ganciclovir during induction therapy in patients with AIDS and newly diagnosed CMV retinitis.
-
▴ During maintenance therapy with valganciclovir, the most commonly reported adverse events included neutropenia, anaemia, thrombocytopenia, gastrointestinal (including diarrhoea, nausea, vomiting and abdominal pain), fever, headache, insomnia, peripheral neuropathy, paraesthesia and retinal detachment.



Similar content being viewed by others
References
Whitley RJ, Jacobson MA, Friedberg DN, et al. Guidelines for the treatment of cytomegalovirus diseases in patients with AIDS in the era of potent antiretroviral therapy: recommendations of an international panel. Arch Intern Med 1998 May 11; 158: 957–69
Akerele T, Lightman S. Current and novel agents for the treatment of cytomegalovirus retinitis. Drugs RD 1999 Nov; 2: 289–97
Anderson RD, Griffy KG, Jung D, et al. Ganciclovir absolute bioavailability and steady-state pharmacokinetics after oral administration of two 3000-mg/d dosing regimens in human immunodeficiency virus- and cytomegalovirus-seropositive patients. Clin Ther 1995; 17(3): 425–32
Markham A, Faulds D. Ganciclovir: an update of its therapeutic use in cytomegalovirus infection. Drugs 1994; 48(3): 455–84
Sugawara M, Huang W, Fei Y-J, et al. Transport of valganciclovir, a ganciclovir prodrug, via peptide transporters PEPT1 and PEPT 2. J Pharm Sci 2000 June; 89(6): 781–9
Roche Laboratories Inc. Prescribing information Valcyte™ (valganciclovir hydrochloride tablets). Nutley, New Jersey, 2001
Noble S, Faulds D. Ganciclovir: an update of its use in the prevention of cytomegalovirus infection and disease in transplant recipients. Drugs 1998; 56(1): 115–46
Jung D, Dorr A. Single-dose pharmacokinetics of valganciclovir in HIV- and CMV-seropositive subjects. J Clin Pharmacol 1999 Aug; 39: 800–4
Brown F, Banken L, Saywell K, et al. Pharmacokinetics of valganciclovir and ganciclovir following multiple oral dosages of valganciclovir in HIV- and CMV-seropositive volunteers. Clin Pharmacokinet 1999 Aug; 37: 167–76
Pescovitz MD, Rabkin J, Merion RM, et al. Valganciclovir results in improved oral absorption of ganciclovir in liver transplant recipients. Antimicrob Agents Chemother 2000 Oct; 44: 2811–5
Martin D, Sierra-Madero J, Walmsley S, et al. Valganciclovir (VGCV) vs IV ganciclovir (GCV) as induction therapy for newly diagnosed cytomegalovirus (CMV) retinitis: a randomised, controlled study [abstract no. 231]. 7th Conference on Retroviruses and Opportunistic Infections; 2000 Jan 30–Feb 2; San Francisco (CA)
Walmsley S, Tseng A. Comparative tolerability of therapies for cytomegalovirus retinitis. Drug Saf 1999; 21(3): 203–24
Jacobson MA. Current management of cytomegalovirus disease in patients with AIDS. AIDS Res Hum Retroviruses 1994 Aug; 10: 917–23
Skiest DJ. Cytomegalovirus retinitis in the era of highly active antiretroviral therapy (HAART). Am J Med Sci 1999 May; 317: 318–35
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Curran, M., Noble, S. Valganciclovir. Drugs 61, 1145–1150 (2001). https://doi.org/10.2165/00003495-200161080-00013
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200161080-00013