Summary
Abstract
Oseltamivir is a prodrug of oseltamivir carboxylate (Ro 64-0802, GS4071), a potent and selective inhibitor of the neuraminidase glycoprotein essential for replication of influenza A and B viruses.
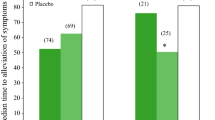
Studies in volunteers with experimental human influenza A or B showed that administration of oral oseltamivir 20 to 200mg twice daily for 5 days reduced both the quantity and duration of viral shedding compared with placebo. Subsequent assessment of the drug at a dosage of 75mg twice daily for 5 days in otherwise healthy adults with naturally acquired febrile influenza showed that oseltamivir reduced the duration of the disease by up to 1.5 days and the severity of illness by up to 38% compared with placebo when initiated within 36 hours of symptom onset (earlier initiation of therapy was associated with faster resolution). The incidence of secondary complications and the use of antibacterials were also reduced significantly in oseltamivir recipients.
A liquid formulation of oseltamivir (2 mg/kg twice daily for 5 days) has been shown to be effective in the treatment of children with influenza, and data presented in abstracts suggest that the drug may also be of use in high-risk populations such as the elderly or those with chronic cardiac or respiratory disease.
In addition to treatment efficacy, the drug has demonstrated efficacy when used for seasonal or household prophylaxis. Oral oseltamivir (75mg once or twice daily for 6 weeks) during a period of local influenza activity significantly prevented the development of naturally acquired influenza by >70% compared with placebo in unvaccinated otherwise healthy adults. The drug also demonstrated efficacy when used adjunctively in previously vaccinated high-risk elderly patients (92% protective efficacy). Short term administration of oseltamivir (75mg once daily for 7 days) may significantly reduce the risk of illness in household contacts of infected persons when administered within 48 hours of symptom onset in the infected person.
Oseltamivir 75mg twice daily for 5 days was well tolerated in clinical trials in healthy adults and high-risk patients, with nausea and vomiting being the most commonly reported events. Gastrointestinal events were mild and transient and both nausea and vomiting were less likely when oseltamivir was taken with food.
Conclusions: Oseltamivir is a well tolerated orally active neuraminidase inhibitor which significantly reduces the duration of symptomatic illness and hastens the return to normal levels of activity when initiated promptly in patients with naturally acquired influenza. It therefore represents a useful therapeutic alternative to zanamivir (especially in patients who prefer oral administration or who have an underlying respiratory disorder) and the M2 inhibitors amantadine and rimantadine (because of its broader spectrum of anti-influenza activity and lower likelihood of resistance) in patients with influenza. In addition, although annual vaccination remains the best means of influenza prevention, there may be a place for oseltamivir in providing household prophylaxis or adjunctive prophylaxis in high-risk vaccinated patients during an outbreak of the disease or for use in patients in whom vaccination is unsuitable or ineffective.
Pharmacodynamic Properties
Oseltamivir carboxylate (Ro 64-0802, GS4071), the active metabolite of oseltamivir, was a potent and selective inhibitor of neuraminidase (an influenza virus surface glycoprotein essential for influenza A and B virus replication) in cultured Madin-Darby canine kidney cells. IC50 values (50% inhibitory concentrations) for oseltamivir carboxylate ranged from 0.0006 to 26.0 µmol/L for laboratory strains of influenza A and B virus and were similar to those reported with zanamivir (a neuraminidase inhibitor which has previously been shown to be a more potent inhibitor of influenza A and B strains than the antiviral agents amantadine, rimantadine and ribavirin).
Oseltamivir carboxylate was at least 106-fold more selective for influenza virus neuraminidases than for parainfluenza virus, Newcastle disease virus, Vibrio cholerae, Clostridium perfringens and human liver microsomes in vitro.
Consistent with in vitro findings, oral oseltamivir demonstrated antiviral activity against influenza A and influenza B viral strains in animal studies (mice and ferrets) and volunteers with experimentally-induced influenza. The drug reduced the quantity and duration of viral shedding compared with placebo in volunteers with either strain of the virus.
Viral resistance to oseltamivir carboxylate has emerged in vitro after sequential passages of influenza A (H3N2) virus in cell culture, but the likelihood of in vivo resistance to oseltamivir appears to be low (only a few cases of drug resistance have been reported in clinical trials). Finally, the drug appears to have low cytotoxicity relative to its antiviral activity, and does not alter the human immune response to influenza.
Pharmacokinetic Profile
Oseltamivir is rapidly absorbed from the gastrointestinal tract after oral administration and is then extensively metabolised, predominantly by hepatic esterases, to its only active metabolite oseltamivir carboxylate. Plasma concentrations of oseltamivir carboxylate were detected within 30 minutes of an oral oseltamivir dose in volunteers and peaked within ≈3 to 4 hours at steady state.
Oseltamivir has high (79%) oral bioavailability relative to an intravenous dose of oseltamivir carboxylate and its absorption is not significantly affected by the presence of food.
Oseltamivir carboxylate was rapidly distributed to the primary site of influenza virus replication (surface epithelial cells of the respiratory tract) after oral administration of oseltamivir in preclinical studies, and to the middle ear and sinuses in volunteers. Oseltamivir carboxylate is eliminated by a first-order process, primarily by glomerular filtration and renal tubular secretion and has a terminal elimination half-life of 6 to 10 hours.
Clearance of oseltamivir carboxylate was slower in the elderly (≥65 years) and faster in children (≤12 years) than in adults. In addition, clearance of oseltamivir carboxylate was reduced in patients with severe renal dysfunction.
Clinically significant drug interactions with oseltamivir are unlikely.
Therapeutic Efficacy
Treatment: In otherwise healthy adults, oral oseltamivir 75mg twice daily for 5 days significantly reduced the duration of naturally acquired febrile influenza by 1.3 to 1.5 days (p < 0.05) and illness severity by up to 38% (p ≤0.01) compared with placebo when initiated in the early stages of infection (i.e. within 36 hours of symptom onset). Furthermore, the incidence of secondary complications (i.e. otitis media, sinusitis, bronchitis and pneumonia), and the use of antibacterials, were significantly reduced by oseltamivir compared with placebo. A higher dosage of oseltamivir (150mg twice daily for 5 days) did not appear to have any further impact on efficacy end-points. Evidence suggests that earlier initiation of therapy is associated with faster resolution of symptoms.
Oseltamivir (2 mg/kg twice daily for 5 days) significantly reduced the median duration of illness and the severity of illness compared with placebo in children (aged ≥1 year) with influenza in 1 well-controlled study. Data presented in abstracts indicate the drug may also be effective in the treatment of influenza in high-risk populations, such as the elderly or those with chronic cardiac or respiratory disease.
Prophylaxis: Evidence from 1 study suggests that oral administration of oseltamivir 75mg once or twice daily for 6 weeks during a period of local influenza virus activity significantly prevents the development of naturally acquired influenza compared with placebo in un vaccinated, otherwise healthy individuals. The drug afforded protection in >70% of exposed patients during a 6-week treatment period. Prophylaxis with oseltamivir 75mg once daily for 6 weeks was also effective when used as adjunctive therapy in vaccinated high-risk elderly patients (92% protective efficacy).
When administered promptly for household prophylaxis (i.e. starting within 48 hours of symptom onset in an infected person), 1 week’s treatment with oseltamivir 75mg once daily significantly reduced the risk of illness by 89% compared with placebo in household contacts.
Tolerability
Oseltamivir is generally well tolerated by patients with influenza. The most common adverse events reported in clinical trials of oral oseltamivir 75mg twice daily for 5 days in adults (n = 1440) were nausea (9.9%) and vomiting (9.4%). Corresponding rates in placebo recipients were 5.6 and 2.9%, respectively. Tolerability-related withdrawals were low (<4%) and similar to those reported with placebo. Episodes of nausea were mild and transient and gastrointestinal upset was reduced when the drug was given with food.
No serious adverse events or significant changes in laboratory parameters occurred during clinical trials of oseltamivir in adults, and no additional treatment-related adverse events emerged during longer term (6-week) administration.
The tolerability profiles of therapeutic dosages of oseltamivir in elderly patients and children appear to be similar to that in otherwise healthy adults.
Dosage and Administration
Oseltamivir is recommended for the treatment of influenza infection in patients of all ages and for prophylaxis against influenza infection in adolescents and adults. The recommended dosage of oseltamivir for the treatment of acute influenza infection in adults and adolescents is 75mg twice daily (with or without food) for 5 days starting within 2 days of symptom onset.
Dosage reduction to 75mg once daily is recommended in patients with creatinine clearance <1.8 L/h (<30 ml/min) and further caution is advised in patients with creatinine clearance <0.6 L/h (<10 ml/min). No dosage adjustment is necessary in the elderly.
An oseltamivir 12 mg/ml oral suspension is available for use in children (aged ≥1 year) with influenza (or adults who cannot swallow a capsule); dosage is adjusted according to bodyweight and ranges from 30mg twice daily for 5 days in children weighing <15kg to 75mg twice daily for 5 days in those over 40kg. There are no current recommendations for the use of oseltamivir in the treatment of influenza in patients with hepatic impairment.
For prophylaxis against influenza infection in individuals aged ≥13 years, a 75mg once daily dosage is recommended (for at least 7 days in those exposed to an infected patient in the past 2 days, or for as long as necessary during a community outbreak).










Similar content being viewed by others
References
Cox NJ, Subbarao K. Influenza. Lancet 1999; 354(9186): 1277–82
Centers for Disease Control and Prevention. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2000; 49 (RR-03), 1–38. Available from: URL: http://www.cdc.gov/epo/mmwr/preview/mmwrhtml/rr4903al.htm [Accessed 2000 Sep 6]
Musich SA, Burton WN, Edington DW. Costs and benefits of prevention and disease management. Dis Manage Health Outcomes 1999 Mar; 5: 153–66
Gubareva LV, Kaiser L, Hayden FG. Influenza virus neuraminidase inhibitors. Lancet 2000 Mar 4; 355: 827–35
Cianci C, Krystal M. Development of antivirals against influenza. Expert Opin Invest Drag 1998 Feb; 7: 149–65
Calfee DP, Hayden FG. New approaches to influenza chemotherapy. Neuraminidase inhibitors. Drugs 1998 Oct; 56: 537–53
Colacino JM, Staschke KA, Laver WG. Approaches and strategies for the treatment of influenza virus infections. Antiviral Chem Chemother 1999; 10(4): 155–85
Cass LM, Efthymiopoulos C, Bye A. Pharmacokinetics of zanamivir after intravenous, oral, inhaled or intranasal administration to healthy volunteers. Clin Pharmacokinet 1999; 36 Suppl. 1: 1–11
Dunn CJ, Goa KL. Zanamivir: a review of its use in influenza. Drugs 1999 Oct; 58: 761–84
Bardsley-Elliot A, Noble S. Oseltamivir. Drags 1999 Nov; 58: 851–60
Mendel DB, Tai CY, Escarpe PA, et al. Oral administration of a prodrug of the influenza viras neuraminidase inhibitor GS 4071 protects mice and ferrets against influenza infection. Antimicrob Agents Chemother 1998 Mar; 42: 640–6
Sidwell RW, Huffman JH, Barnard DL, et al. Inhibition of influenza virus infections in mice by GS4104, an orally effective influenza virus neuraminidase inhibitor. Antiviral Res 1998 Feb; 37: 107–20
Hayden FG, Treanor JJ, Fritz RS, et al. Use of the oral neuraminidase inhibitor oseltamivir in experimental human influenza: randomized, controlled trials for prevention and treatment. JAMA 1999 Oct 6; 282(13): 1240–6
Hayden FG, Jennings L, Robson R, et al. Oral oseltamivir in human experimental influenza B infection. Antiviral Therapy 2000; 5(3): 205–13
Nicholson KG, Aoki FY, Osterhaus A, et al. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Lancet 2000 May 27; 355: 1845–50
Treanor JJ, Hayden FG, Vrooman PS, et al. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. JAMA 2000 Feb 23; 283: 1016–24
Robson R, Saiedabadi N, Ward P. Oral oseltamivir reduces the duration of influenza illness by 2.7 days in previously healthy adults [abstract, pg 184]. 9th International Congress on Infectious Diseases; 10–13 Apr 2000; Buenos Aires
Hayden FG, Atmar RL, Schilling M, et al. Use of the selective oral neuraminidase inhibitor oseltamivir to prevent influenza. N Engl J Med 1999 Oct 28; 341: 1336–43
Mendel DB, Sidwell RW. Influenza virus resistance to neuraminidase inhibitors. Drug Resist Update 1998; 1(3): 184–9
Sidwell RW, Bailey KW, Bemis PA, et al. Influence of treatment schedule and viral challenge dose on the in vivo influenza virus-inhibitory effects of the orally administered neuraminidase inhibitor GS 4104. Antivir Chem Chemother 1999 Jul; 10: 187–93
Mendel DB, Tai CY, Escarpe PA. In vitro selection and characterization of a human influenza virus with decreased susceptibility to GS 4071 [abstract]. Antiviral Res 1998 Mar; 37: A71
Tai CY, Escarpe PA, Sidwell RS, et al. Characterization of human influenza virus variants selected in vitro in the presence of the neuraminidase inhibitor GS 4071. Antimicrob Agents Chemother 1998; 42(12): 3234–41
Sidwell RW, Bailey KW, Wong MH, et al. Lack of in vivo development of influenza A virus resistance to the orally effective neuraminidase inhibitor GS4104 [abstract no. 112]. Antiviral Res 1998 Mar; 37: A71
Covington E, Mendel DB, Escarpe P, et al. Phenotypic and genotypic assay of influenza virus neuraminiase indicates a low incidence of viral drug resistance during treatment with oseltamivir [abstract]. II International Symposium on Influenza and other Respiratory Viruses; Dec 10–12 1999, Grand Cayman
Roche Laboratories Inc. Tamiflu™ (oseltamivir phosphate): capsules and for oral suspension prescribing information. Available from: URL: http://www.rocheusa.com/products/tamiflu/pi.html [Accessed 2000 Dec 21]
Gubareva LV, Bethell R, Hart GJ, et al. Characterization of mutants of influenza A viras selected with the neuraminidase inhibitor 4-guanidino-Neu5Ac2en. J Virol 1996;70(3): 1818–27
Staschke KA, Colacino JM, Baxter AJ, et al. Molecular basis for the resistance of influenza viruses to 4-guanidino-Neu5Ac2en. Virology 1995 Dec 20; 214(2): 642–6
Burger RA, Billingsley JL, Huffman JH, et al. Immunological effects of the orally administered neuraminidase inhibitor oseltamivir in influenza virus-infected and uninfected mice. Immunopharmacology 2000; 47(1): 45–52
Massarella JW, He GZ, Dorr A, et al. The pharmacokinetics and tolerability of the oral neuraminidase inhibitor oseltamivir (Ro 64-0796/GS4104) in healthy adult and elderly volunteers. J Clin Pharmacol 2000; 40(8): 836–43
He G, Massarella J, Ward P. Clinical pharmacokinetics of the prodrug oseltamivir and its active metabolite Ro 64-0802. Clin Pharmacokinet 1999 Dec; 37: 471–84
Oo C, Barrett J, Mann J, et al. Pharmacokinetics of the oral neuraminidase inhibitor oseltamivir in pediatric patients [abstract]. Clin Microbiol Infect 2000; 6 Suppl. 1: 202
He G, Massarella J, Schulz R, et al. The effect of food on the pharmacokinetics of the novel oral neuraminidase inhibitor RO 64-0796/GS4104 [poster]. American Association of Pharmaceutical Scientists, Southeast Regional Meeting; 1999 Jun 21; Durham, (NC)
Kurowski M, Barrett J, Waalberg E, et al. Oral oseltamivir rapidly delivers active drug levels to middle ear and sinuses in humans [poster]. ICAAC, 17–20 Sep 2000; Toronto
Eisenberg EJ, Bidgood A, Cundy KC. Penetration of GS4071, a novel influenza neuraminidase inhibitor, into rat bronchoalveolar lining fluid following oral administration of the prodrug GS4104. Antimicrob Agents Chemother 1997 Sep; 41: 1949–52
Wiltshire HR, Muir J, Lambkin R, et al. Distribution of the influenza neuraminidase inhibitor GS4071 in ferrets, following oral administration of its prodrug GS4104 [poster]. Meeting of the European Society for Clinical Virology (ESCV); 1998 Aug 30–Sep 2; Hamburg
He G, Massarella J, Robson R, et al. The pharmacokinetics and tolerability of the oral neuraminidase inhibitor Ro 64-0796 in subjects with renal impairment [poster]. 9th ECCMID; 1999 Mar 21–24; Berlin
He G, Massarella J, Aitken M, et al. The pharmacokinetics and safety of the oral neuraminidase inhibitor RO 64-0796/GS4104 when administered concurrently with cimetidine or probenecid in healthy subjects [abstractno.p17(plus poster)]. J Antimicrob Chemother 1999 Jul; 44 Suppl. A: 44
He G, Massarella J, Aitken M, et al. The safety and pharmacokinetics of the neuraminidase inhbitor Ro 64-0796 when administered concurrently with paracetamol [abstract no P247]. Clin Microbiol Infect 1999; 5 Suppl. 3: 150
Barrett J, Oo C, He G, et al. Lack of pharmacokinetic interaction between the oral neuraminidase inhibitor prodrug oseltamivir and amoxicillin. F. Hoffmann-La Roche; report no. ROCAOUIN0106. (Data on file)
An open-label, relative bioavailability study of the market oral suspension (/V37), the clinical trial oral suspension (/V20-01) and the market capsule formulations (/V22) in healthy volunteers. F. Hoffmann-La Roche; protocol no. WP16225. (Data on file)
Whitley RJ, Hayden FG, Reisinger KS, et al. Oral oseltamivir treatment of influenza in children. Pediatr Infect Dis J 2001; 20: 127–33
Zaug M, Mahoney P, Ward P, et al. Oral oseltamivir is effective in the treatment of acute influenza in a vaccinated population [abstract]. Clin Microbiol Infect 2000; 6 Suppl. 1: 139
Torres F, Madinger N, Zamora M, et al. Treatment of influenza A and B lower respiratory tract infections with oseltamivir in solid organ and bone marrow transplant recipients [abstract]. Am J Respir Crit Care Med 2000 Mar; 161 (3 Pt 2) Suppl.: A720
Peters PH, Gravenstein S, Norwood P, et al. Long term use of oseltamivir for the prophylaxis of influenza in a vaccinated frail elderly population. J Am Geriatr Soc 2001. In press
Welliver R, Monto AS, Carewicz O, et al. Effectiveness of oseltamivir in preventing influenza in household contacts: a randomised controlled trial. JAMA 2001. In press
Aoki F, Macleod M, Paggiaro P, et al. Maximising benefits of influenza treatment with oral oseltamivir: results of the IMPACT study [poster]. ICAAC, 2000 Sep 17–20; Toronto
Kinnersley N, Struthers L, Ward P. Impact of endpoint definition on efficacy in studies with oseltamivir [poster]. II International Symposium on Influenza and other Respiratory Viruses 1999; Dec 10–12 Grand Cayman
Winther B, Hayden FG, Whitley R, et al. Oral oseltamivir reduces the risk of developing acute otitis media following influenza infection in children [poster]. ICAAC; 2000 Sep 17–20; Toronto
Zaug M, Mahoney P, Ward P, et al. Oral oseltamivir is effective in the treatment of acute influenza in a vaccinated population [poster]. ECCMID; 2000 May 28–31; Stockholm
Nichol KL, Margolis KL, Wuorenma J, et al. The efficacy anad cost effectiveness of vaccination against influenza among elderly persons living in the community. N Engl J Med 1994; 331: 778–84
Mullooly JP, Bennett MD, Hornbrook MC, et al. Influenza vaccination programs for elderly persons: cost-effectiveness in a health maintenance organization. Ann Intern Med 1994; 121: 947–52
Hall CB, Dolin R, Gala CL, et al. Children with influenza A infection: treatment with rimantadine. Pediatrics 1987 Aug; 80(2): 275–82
Hayden FG, Sperber SJ, Belshe RB, et al. Recovery of drug-resistant influenza A virus during therapeutic use of rimantadine. Antimicrob Agents Chemother 1991; 35(9): 1741–7
Hayden FG, Belshe RB, Clover RD, et al. Emergence and apparent transmission of rimantadine-resistant influenza A virus in families. N Engl J Med 1989; 321: 1696–702
Mast EE, Harmon MW, Gravenstein S, et al. Emergence and possible transmission of amantadine-resistant viruses during nursing home outbreaks of influenza A (H3N2). Am J Epidemiol 1991; 134: 988–97
Laver WG, Kelly GD. Influenza —a new problem? [letter]. Med J Aust 1999 Nov 1; 171: 504
Long JK, Mossad SB, Goldman MP. Antiviral agents for treating influenza. Cleve Clin J Med 2000 Feb; 67: 92–5
Snacken R, Influenza Diagnosis Working Party. Managing influenza in primary care: a practical guide to clinical diagnosis. Dis Manage Health Outcomes 2000 Aug; 8(2): 79–85
Glaxo Wellcome Inc. RELENZA (zanamivir for inhalation). North Carolina: Glaxo Wellcome Inc., Apr 2000
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: F.Y. Aoki, University of Manitoba, Winnipeg, Manitoba, Canada; D.M. Fleming, Birmingham Research Unit of the Royal College of General Practitioners, Harborne, Birmingham, England; A.S. Monto, School of Public Health, University of Michigan, Ann Arbor, Michigan, USA; K.G. Nicholson, Leicester Royal Infirmary, Leicester, England; C.E. Nord, Karolinska Institute, Huddinge University Hospital, Huddinge, Sweden; K.S. Reisinger, Primary Physicians Research, Pittsburgh, Pennsylvania, USA; M. Schilling, University of Iowa College of Medicine, Iowa City, Iowa, USA; S.D. Shafran, University of Alberta, Edmonton, Alberta, Canada; R.W. Sidwell, Institute for Antiviral Research, Utah State University, Logan, Utah, USA; J.J. Treanor, Infectious Diseases Unit, University of Rochester, Rochester, New York, USA.
Data Selection
Sources: Medical literature published in any language since 1966 on oseltamivir, identified using Medline and EMBASE, supplemented by AdisBase (a proprietary database of Adis International, Auckland, New Zealand). Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: Medline search terms were ‘oseltamivir’ or ‘EN 241104’ or ‘GS 4104’ or ‘RO 640796’. EMBASE search terms were ‘oseltamivir’ or ‘GS 4104’ or ‘RO640796’. AdisBase search terms were ‘oseltamivir’ or ‘EN 241104’ or ‘GS4104’ or ‘RO 640796’. Searches were last updated 15 January, 2001.
Selection: Studies in patientswith influenza Aor influenzaBwho received oseltamivir, and studies inwhich the drugwas given prophylactically. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Oseltamivir, influenza A, influenza B, treatment, prophylaxis, pharmacodynamics, pharmacokinetics, therapeutic use, tolerability, dosage and administration.
Rights and permissions
About this article
Cite this article
McClellan, K., Perry, C.M. Oseltamivir. Drugs 61, 263–283 (2001). https://doi.org/10.2165/00003495-200161020-00011
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200161020-00011