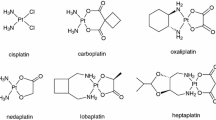
Abstract
We review the pharmacology and clinical administration of the commonly used platinum-based anticancer drugs cisplatin and carboplatin, and the more recently approved diamminocyclohexane-based oxaliplatin. The development of analogues of cisplatin has been focused upon identifying compounds with less toxicity and with a different spectrum of activity.
Carboplatin exemplifies the former, while the initial data with oxaliplatin support its activity in cisplatin-resistant tumours. The clinical pharmacokinetics of the drugs are reviewed. Incorporation of these data into the design of clinical regimens has permitted individualised therapy with carboplatin, and has enhanced safety. Additional investigation of the pharmacodynamics of all of these agents is expected to result in their selective application. The clinical effects of these analogues are discussed.



Similar content being viewed by others
References
Loehrer P, Einhorn L. Drugs five years later. Cisplatin. Ann Intern Med 1984; 100: 704–13
Cvitkovic E, Spaulding J, Bethune V, et al. Improvement of cis-dichlorodiammineplatinum (NSC 119875): therapeutic index in an animal model. Cancer 1977; 39: 1357–61
Hayes D, Cvitkovic E, Golbey R, et al. High dose cis-platinum diammine dichloride: amelioration of renal toxicity by mannitol diuresis. Cancer 1977; 39: 1372–81
Gralla RJ, Osoba D, Kris MG, et al. Recommendations for the use of antiemetics: evidence-based, clinical practice guidelines. American Society Clinical Oncology. J Clin Oncol 1999; 17: 2971–94
Connors T, Jones M, Ross W, et al. New platinum complexes with anti-tumour activity. Chem Biol Interact 1972; 5: 415–24
Harrap K. Preclinical studies identifying carboplatin as a viable cisplatin alternative. Cancer Treat Rev 1985; 12: A21–A33
Calvert AH, Newell DR, Gumbrell LA, et al. Carboplatin dosage: prospective evaluation of a simple formula based on renal function. J Clin Oncol 1989; 7: 1748–56
Alberts DS, Green S, Hannigan EV, et al. Improved therapeutic index of carboplatin plus cyclophosphamide vs cisplatin plus cyclophosphamide: final report by the Southwest Oncology Group of a phase III randomized trial in stages 3 and 4 ovarian cancer. J Clin Oncol 1992; 10: 706–17
Bajorin DF, Sarosdy MF, Pfister DG, et al. Randomized trial of etoposide and cisplatin versus etoposide and carboplatin in patients with good-risk germ cell tumours: a multiinstitutional study. J Clin Oncol 1993; 11(4): 598–606
De Andres L, Brunet J, Lopez-Pousa A, et al. Randomized trial of neoadjuvant cisplatin and fluorouracil versus carboplatin and fluorouracil in patients with stage IV-MO head and neck cancer. J Clin Oncol 1995; 13: 1493–500
Burchenal JH, Irani G, Kern K, et al. 1,2-diaminocyclohexane platinum derivatives of potential clinical value. Recent Results Cancer Res 1980; 74: 146–55
Chaney SG. The chemistry and biology of platinum complexes with the 1,2-diaminocyclohexane carrier ligand. Int J Oncol 1995; 6: 1291–305
Extra JM, Espic M, Calvo F, et al. Phase I study of oxaliplatin in patients with advanced cancer. Cancer Chemother Pharmacol 1990; 25: 299–303
Tashiro R, Kawada Y, Sakuri Y, et al. Antitumor activity of a new platinum complex, oxalato (trans-I-1,2-diaminocyclohexane) platinum (II): new experimental data. Biomed Pharmacother 1989; 43: 251
Mathe G, Kidani Y, Sekiguchi M, et al. Oxalato-platinum or 1-OHP, a third-generation platinum complex: an experimental and clinical appraisal and preliminary comparison with cisplatinum and carboplatinum. Biomed Pharmacother 1989; 43: 237–50
Jennerwein MM, Eastman A, Khokhar A. Characterization of adducts produced in DNA by isomeric 1,2-diaminocyclohexane platinum (II) complexes. Chem Bil Interact 1989; 70: 39–49
Raymond E, Chaney SG, Taamma A, et al. Oxaliplatin: a review of preclinical and clinical studies. Ann Oncol 1998; 9(10): 1053–71
Saris CP, van de Vaart PJ, Rietbroek R, et al. In vitro formation of DNA adducts by cisplatin, lobaplatin and oxaliplatin in calf thymus DNA in solution and in cultured human cells. Carcinogenesis 1996; 17(12): 2763–9
Kraker AJ, Moore CW. Accumulation of cis-diamminedichloroplatinum (II) and platinum analogues by platinumresistant murine leukemia cells in vitro. Cancer Res 1988; 48: 9–13
Rixe O, Ortuzar W, Alvarez M, et al. Oxaliplatin, tetraplatin, cisplatin, and carboplatin: spectrum of activity in drug-resistant cell lines and in the cell lines of the National Cancer Institute's Anticancer Drug Screen panel. Biochem Pharmacol 1996; 52(12): 1855–65
Canetta R, Bragman K, Smaldone L, et al. Carboplatin: current status and future prospects. Cancer Treat Rev 1988; 15 Suppl. B: 17–32
Judson I, Kelland LR. New developments and approaches in the platinum arena. Drugs 2000; 59 Suppl. 4: 29–36
Duffull S, Robinson B. Clinical pharmacokinetics and dose optimisation of carboplatin. Clin Pharmacokinet 1997; 33: 161–83
van der Vijgh W. Clinical pharmacokinetics of carboplatin. Clin Pharmacokinet 1991; 21: 242–61
Extra J, Marty M, Brienza S, et al. Pharmacokinetics and safety profile of oxaliplatin. Semin Oncol 1998; 25: 13–22
DeConti R, Toftness B, Lange R, et al. Clinical and pharmacological studies with cis-diamminedichloroplatinum (II). Cancer Res 1973; 33: 1310–5
Himmelstein K, Patton T, Belt R, et al. Clinical kinetic s on intact cisplatin and some related species. Clin Pharmacol Ther 1981; 29: 658–64
Vermorken J, van der Vijgh W, Klein I, et al. Pharmacokinetics of free and total platinum species after short-term infusion of cisplatin. Cancer Treat Rep 1984; 68: 505–13
Gormley P, Bull J, LeRoy A, et al. Kinetics of cisdichlorodiammineplatinum. Clin Pharmacol Ther 1979; 25: 351–7
Belt R, Himmelstein K, Patton T, et al. Pharmacokinetics of non-protein-bound platinum species following administration of cis-dichlorodiammineplatinum(II). Cancer Treat Rep 1979; 63: 1515–21
Harland S, Newell D, Siddik Z, et al. Pharmacokinetics of cisdiammine-1,1-cyclobutane dicarboxylate platinum(II) in patients with normal and impaired renal function. Cancer Res 1984; 44: 1693–7
Calvert A, Newell D, Gumbrell L, et al. Carboplatin dosage: prospective evaluation of a simple formula based on renal function. J Clin Oncol 1989; 7: 1748–56
Gamelin E, Bouil A, Boisdron-Celle M, et al. Cumulative pharmacokinetic study of oxaliplatin, administered every three weeks, combined with 5-fluorouracil in colorectal cancer patients. Clin Cancer Res 1997; 3: 891–9
Egorin MJ, Van Echo DA, Olman EA, et al. Prospective of validation of a pharmacologically-based dosing scheme for the cis-diamminedichloroplatin (II) analogue cis-diamminecyclobutanedicarboxylato platinum (II). Cancer Res 1985; 45: 6502–6
Calvert AH, Newell DR, Gumbrell LA, et al. Carboplatin dosage: prospect of evaluation of a simple formula based on renal function. J Clin Oncol 1989; 7: 1748–56
Chatelut E, Canal P, Brunner V, et al. Prediction of carboplatin clearance from standard morphological and biological patient characteristics. J Natl Cancer Inst 1995; 87: 573–80
Jodrell D, Egorin M, Canetta R, et al. Relationships between carboplatin exposure and tumor response and toxicity in patients with ovarian cancer. J Clin Oncol 1992; 10: 520–8
Reyno L, Egorin M, Canetta R, et al. Impact of cyclophosphamide on relationships between carboplatin exposure and response or toxicity when used in the treatment of advanced ovarian cancer. J Clin Oncol 1993; 11: 1156–64
O'Dwyer PJ, Hamilton TC, Yao K-S, et al. Cellular pharmacodynamics of anticancer drugs. In: Schilsky R, Milano G, Ratain M, editors. Cancer pharmacology. 1996: 329–62
Reed E, Ozols RF, Tarone R, et al. Platinum-DNA adducts in leucocyte DNA correlate with disease response in ovarian cancer patients receiving platinum-based chemotherapy. Proc Natl Acad Sci USA 1987; 84: 5024–8
Schellens JHM, Ma J, Planting ASTL, et al. Relationship between the exposure to cisplatin, DNA-adduct formation in leucocytes, and tumor response in patients with solid tumors. Br J Cancer 1996; 73: 1569–75
Schellens JHM, Ma J, Planting ASTL, et al. Adaptive intrapatient dose escalation of cisplatin. Proc Am Soc Clin Oncol 1996; 15: 178
Ozols RF, Ostchega Y, Myers CE, et al. High-dose cisplatin in hypertonic saline in refractory ovarian cancer. J Clin Oncol 1985; 3: 1246–50
Howell SB, Pfeifle CE, Wung WE, et al. Intraperitoneal Cisdiamminedichloroplatin with synthetic thiosulfate protection. Cancer Res 1983; 43: 1426–31
Alberts DS, Liu PY, Hannigan EV, et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med 1996; 335: 1950–5
Solomon B, Soulen MC, Baum RA, et al. Chemoembolization of hepatocellular carcinoma with cisplatin, doxorubicin, mitomycin-C, ethiodol, and polyvinyl alcohol: prospective evaluation of response and survival in a U.S. population. J Vasc Intervent Radiol 1999; 10: 793–8
Evans BD, Raju KS, Calvert AH, et al. Phase II study of JM8, a new platinum analog, in ovarian cancer. Cancer Treat Rep 1983; 67: 997–1000
Ozols RF, Behrens BC, Ostchega Y, et al. High dose cisplatin and high dose carboplatin in refractory ovarian cancer. Cancer Treat Rev 1985; 12: 59–65
Levi F, Giachetti S, Adam R, et al. Chronomodulation of chemotherapy against metastatic colorectal cancer. Eur J Cancer 1995; 31A: 1264–70
McMahon SB, Priestley JV. Peripheral neuropathies and neurotrophic factors. Animal models and clinical perspectives. Curr Opin Neurobiol 1995; 5: 616–24
Langer CJ, Leighton JC, Comis RL, et al. Paclitaxel and carboplatin in the treatment of advanced non-small cell lung cancer. J Clin Oncol 1995; 13: 1860–70
Brienza S, Vignoud J, Itzhaki M, et al. Oxaliplatin (L-OHP): Global safety in 682 patients. Proc Am Soc Clin Oncol 1995; 14: 209
Extra J, Espie M, Calvo F, et al. Phase I study of oxaliplatin in patients with advanced cancer. Cancer Chemother Pharmacol 1990; 25: 299–303
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
O'Dwyer, P.J., Stevenson, J.P. & Johnson, S.W. Clinical Pharmacokinetics and Administration of Established Platinum Drugs. Drugs 59 (Suppl 4), 19–27 (2000). https://doi.org/10.2165/00003495-200059004-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200059004-00003