Abstract
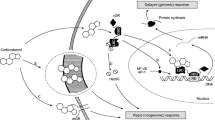
Current guidelines on the management of childhood asthma have emphasised the important preventive role of inhaled corticosteroids, which should be used at the lowest possible doses that are compatible with good disease control. However, some children do not respond to inhaled corticosteroids, the most common reasons for which are inability to use conventional hand-held inhalers (plus spacers and face masks) effectively or lack of cooperation with them, particularly among infants and young children. In these patients, nebulisers have proved effective in administering corticosteroids, and this form of delivery is often preferred by both the children and their parents, despite their longer administration times (commonly around 10 minutes). Compliance with these devices may therefore be better than with a conventional pressurised metered-dose inhaler plus spacer and face mask.
Recent studies with nebulised budesonide have demonstrated that once-daily administration is as effective in maintaining control of asthma symptoms in children as the usual twice-daily administration. In children with moderately severe persistent asthma, the improvement provided by once-daily nebulised doses of 1.0mg budesonide has been found to be equivalent to that with twice-daily doses of 0.25 or 0.5mg, indicating that once-daily therapy is an effective option that can be considered in many patients. In view of the time-consuming nature of nebuliser administration, reduction of the frequency of corticosteroid administration from twice to once daily may be useful in simplifying the treatment programme and improving compliance with it. This may be beneficial in reducing under-utilisation of inhaled corticosteroids in children with asthma and improving long term control of the disease.
Similar content being viewed by others
References
The British Thoracic Society. The British guidelines on asthma management: 1995 review and position statement. Thorax 1997; 52 Suppl. 1: S1–S21
Warner JO, Naspitz CK, editors. Third International Pediatric Consensus Statement on the management of childhood asthma. Pediatr Pulmonol 1998; 25: 1–17
Brownlee KG. A rationale for the use of nebulized steroids in children. Eur Respir Rev 1997; 7: 177–9
Pedersen S. Inhalers and nebulizers: which to choose and why. Respir Med 1996; 90: 69–77
Powell CVE, Everard ML. Treatment of childhood asthma: options and rationale for inhaled therapy. Drugs 1998; 55: 237–52
Shapiro G, Mendelson L, Kraemer J, et al. Efficacy and safety of budesonide inhalation suspension (Pulmicort Respules) in young children with inhaled steroid-dependent, persistent asthma. J Allergy Clin Immunol 1998; 102: 789–96
British Thoracic Society Nebuliser Project Group. Nebuliser therapy. Guidelines. Thorax 1997; 52 Suppl. 2: S4–S24
O’Callaghan C, Barry PW. The science of nebulised drug delivery. Thorax 1997; 52 Suppl. 2: S31–S44
Smaldone GC, Cruz-Rivera M, Nikander K. In vitro determination of inhaled mass and particle distribution for budesonide nebulizing suspension. J Aerosol Med 1998; 11: 113–25
Nikander K, Turpeinen M, Wollmer P. The conventional ultrasonic nebulizer proved inefficient in nebulizing a suspension. J Aerosol Med 1999; 12: 47–53
Noble V, Ruggins NR, Everard ML, et al. Inhaled budesonide for chronic wheezing under 18 months of age. Arch Dis Child 1992; 67: 285–8
Webb MSC, Milner AD, Hiller EJ, et al. Nebulised beclomethasone dipropionate suspension. Arch Dis Child 1986; 61: 1108–10
Storr J, Lenney CA, Lenney W. Nebulised beclomethasone dipropionate in preschool asthma. Arch Dis Child 1986; 61: 270–3
Pedersen W, Prahl P. Jet-nebulized beclomethasone dipropionate in the management of bronchial asthma. Topical steroids for asthmatic children younger than 4 years. Allergy 1987; 42: 272–5
O’Callaghan C. Particle size of beclomethasone dipropionate produced by two nebulisers and two spacing devices. Thorax 1990; 45: 109–11
Wennergren G, Nordvall SL, Hedlin G, et al. Nebulized budesonide for the treatment of moderate to severe asthma in infants and toddlers. Acta Paediatr 1996; 85: 183–9
de Blic J, Delacourt C, Le Bourgeois M, et al. Efficacy of nebulized budesonide in treatment of severe infantile asthma: a double-blind study. J Allergy Clin Immunol 1996; 98: 14–20
Ilangovan P, Pedersen S, Godfrey S, et al. Treatment of severe steroid dependent preschool asthma with nebulised budesonide suspension. Arch Dis Child 1993; 68: 356–9
Georgitis J. A study of once-a-day budesonide nebulizing suspension (BNS) and placebo (PBO) in asthmatic children aged six to eight years [abstract]. Chest 1997; 112 Suppl.: 37S
Gentile DA, Skoner DP, Walton-Bowen KL, et al. The efficacy of once daily Pulmicort Respules™ (budesonide nebulizing suspension; BNS) and placebo (PBO) in infants and children with persistent asthma [abstract]. Pediatr Res 1998; 43: 332
Baker JW, Mellon M, Wald J, et al. A multiple-dosing, placebo-controlled study of budesonide inhalation suspension given once or twice daily for treatment of persistent asthma in young children and infants. Pediatrics 1999; 103: 414–21
Scott M, Ellis M, Cruz-Rivera M, et al. Improved asthma control with once-daily budesonide inhalation suspension (BIS; Pulmicort Respules™) in children <4 and ≥4 years of age [abstract]. J Allergy Clin Immunol 1999; 103: S130
Mellon M, Leflein J, Walton-Bowen K, et al. The efficacy of budesonide inhalation suspension (BIS) in infants and young children with persistent asthma using face masks or mouth pieces [abstract]. Ann Allergy Asthma Immunol 1999; 82: 99
Barnes PJ, Pedersen S, Busse W. Efficacy and safety of inhaled corticosteroids: new developments. Am J Respir Crit Care Med 1998; 157: S1–S53
Baker J, Yunginger J, Walton-Bowen K, et al. A safety and HPA-axis evaluation of four dose regimens of budesonide nebulizing suspension (BNS; Pulmicort® Respules™) compared to placebo (PBO) in infants and young children with persistent asthma [abstract]. Am J Respir Crit Care Med 1998; 157 Suppl.: 405
Pearlman D. HPA-axis function in asthmatic infants and young children treated with budesonide nebulizing suspension (BNS) or placebo (PBO) once-a-day (QD) [abstract]. Ann Allergy Asthma Immunol 1998; 80: 110
Irani AA, Nayak A, Cruz-Rivera M, et al. HPA-axis function in infants and young children with persistent asthma treated for 52 weeks with budesonide inhalation suspension (BIS; Pulmicort Respules™) [abstract]. J Allergy Clin Immunol 1999; 103: 130–1
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shapiro, G. Once-Daily Inhaled Corticosteroids in Children with Asthma. Drugs 58 (Suppl 4), 43–49 (1999). https://doi.org/10.2165/00003495-199958004-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-199958004-00006