Abstract
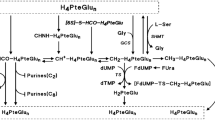
The oral chemotherapeutic agent tegafur/uracil (UFT®) is the first of a new class of anticancer drugs called dihydropyrimidine dehydrogenase inhibitory fluoropyrimidines. Tegafur/uracil combines uracil with the fluorouracil prodrug tegafur in a 4: 1 molar ratio. Uracil competitively inhibits the degradation of fluorouracil, which results in the concentration of fluorouracil remaining at sustained levels in both plasma and tumour. Tegafur/uracil has been commercially available in Japan since 1983 and examined extensively in various tumours. Trials conducted in the US have focused on the combination of tegafur/uracil plus calcium folinate (calcium leucovorin) [ORZEL®]. Several phase I and II trials have evaluated the maximum tolerated dose, pharmacokinetics, efficacy, and safety of this combination in the treatment of colorectal cancer. Results have shown that tegafur/uracil at 300 mg/m2/day in divided doses given every 8 hours for 28 days provides prolonged exposure to fluorouracil. Furthermore, tegafur/uracil + calcium folinate is well tolerated, with dose-limiting toxicity manifesting as diarrhoea. Compared with intravenous fluorouracil plus folinic acid (leucovorin) regimens, tegafur/uracil + calcium folinate has similar efficacy with less toxicity and is more convenient because it is an oral regimen. Early studies have also shown potential cost savings because of fewer complications.
Similar content being viewed by others
References
Landis S, Murray T, Bolden S, et al. Cancer Statistics, 1998. CA Cancer J Clin 1998; 48: 6–29
Moertel C, Fleming T, MacDonald J, et al. Fluorouracil plus levamisole as effective adjuvant therapy after resection of stage III colon carcinoma: a final report. Ann Intern Med 1995; 122: 321–6
Lokich J, Ahlgren J, Gullo J, et al. A prospective randomized comparison of continuous infusion fluorouracil with a conventional bolus schedule in metastatic colorectal carcinoma: a Mid-Atlantic Oncology Program study. J Clin Oncol 1989; 7: 425–32
Leichman C, Fleming T, Muggia F, et al. Phase II study of fluorouracil and its modulation in advanced colorectal cancer: a Southwest Oncology Group study. J Clin Oncol 1995; 13: 1303–11
The Meta-analysis Group in Cancer. Efficacy of intravenous continuous infusion of fluorouracil compared with bolus administration in advanced colorectal cancer. J Clin Oncol 1998; 16: 301–8
Hill M, Norman A, Cunningham D, et al. Impact of protracted venous infusion fluorouracil with or without interferon alpha-2b on tumor response, survival, and quality of life in advanced colorectal cancer. J Clin Oncol 1995; 13: 2317–23
Hiller S, Zhuk R, Lidak M. Analogs of pyridine nucleosides. Dokl Adad Nauk SSSR 1967; 176: 332–5
Friedman M, Ignoffo R. A review of the United States clinical experience of the fluoropyrimidine, ftorafur (NSC-148958). Cancer Treat Rev 1980; 7: 205–13
Buroker T, Padilla F, Groppe C, et al. Phase II evaluation of ftorafur in previously untreated colorectal cancer: a Southwest Oncology Group study. Cancer 1979; 44: 48–51
Valdivieso M, Bodey G, Gottlieb J, et al. Clinical evaluation of ftorafur (pyrimidine-deoxyribose Nl-2′-furanidyl-5-fluorouracil). Cancer Res 1976; 36: 1821–4
Ansfield F, Kallas G, Singson J. Phase I-II studies of oral tegafur (ftorafur). J Clin Oncol 1983; 1: 107–10
Taguchi T. Clinical application of biochemical modulation in cancer chemotherapy: biochemical modulation for 5-FU. Oncology 1997; 54 Suppl. 1: 12–8
Fujii S, Kitano S, Ikenaka K, et al. Studies on coadministration of uracil or cytosine on antitumor activity of FT-207 or 5-FU derivatives. Jpn J Cancer Chemother 1979; 6: 377–84
Fujii S, Ikenaka K, Fukushima M, et al. Effect of coadministration of uracil or cytosine on the anti-tumor activity of clinical doses of l-(2-tetrahydrofuryl)-5-fluorouracil and level of 5-fluorouracil in rodents. Gann 1979; 70: 209–14
Fujii S, Ikenaka K, Fukushima M, et al. Effect of uracil and its derivatives on antitumor activity of 5-fluorouracil and 1-(2-tetrahydrofuryl)-5-fluorouracil. Gann 1978; 69: 763–72
Watanabe H, Yamamoto S, Naito T. Clinical results of oral UFT therapy under cooperative study. Jpn J Cancer Chemother 1980; 7: 1588–96
Taguchi T. Experience with UFT in Japan. Oncology 1997; 11 Suppl. 10: 30–4
Pazdur R, Lassere Y, Diaz-Canton E, et al. Phase I trials of uracil-tegafur (UFT) using 5 and 28 day administration schedules: demonstration of schedule-dependent toxicities. Anti-cancer Drugs 1996; 7: 728–33
Pazdur R, Lassere Y, Rhodes V, et al. Phase II trial of uracil and tegafur plus oral leucovorin: an effective oral regimen in the treatment of metastatic colorectal carcinoma. J Clin Oncol 1994; 12: 2296–300
Au J-S, Sadee W. Activation of ftorafur [R,S-l-(tetrahydro-2-furanyl)-5-fluorouracil] to 5-fluorouracil and y-butyrolactone. Cancer Res 1980; 40 Pt 1: 2814–9
Grem J. 5-Fluoropyrimidines. In: Chabner B, Longo D, editors. Cancer chemotherapy and biotherapy: principles and practice. Philadelphia: Lippincott-Raven, 1996: 149–211
Lockshin A, Danenberg P. Biochemical factors affecting the tightness of 5-fluorodeoxyuridylate binding to human thymidylate synthetase. Biochem Pharmacol 1981; 30: 247–57
Suemasu K, Nomoto C, Higashi Y. Concentrations of 5-FU level in the tissue and blood of patients with breast cancer by preoperative administration of UFT. Gan To Kagaku Ryoho 1982; 9: 667–71
Tsujimoto T, Sakai S, Murata M, et al. Concentrations of 5-FU in the tissue and serum of patients with head and neck malignant tumors by preoperative administration of UFT. Jpn J Cancer Chemother 1983; 10: 78–83
Nakajima O, Ihara K, Isoda T, et al. Phase I and II studies on a mixture of l-(2-tetrahydrofuryl)-5-fluorouracil and uracil (UFT). Cancer Chemother 1980; 7: 1558
Maeda M, Izumi A, Hamazoe R. Intratumorous 5-FU concentrations after coadministration of FT-207 with uracil [in Japanese]. Igaku No Ayumi 1981; 116: 97
Rustum Y. Mechanism-based improvement in the therapeutic selectivity of 5-FU prodrug alone and under conditions of metabolic modulation. Oncology 1997; 54 Suppl. 1: 7–11
Poon M, O’Connell M, Moertel C, et al. Biochemical modulation of fluorouracil: evidence of significant improvement of survival and quality of life in patients with advanced colorectal carcinoma. J Clin Oncol 1989; 7: 1407–18
Advanced Colorectal Cancer Meta-Analysis Project. Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: evidence in terms of response rate. J Clin Oncol 1992; 10: 896–903
Ichikura T, Tomimatsu S, Okusa Y, et al. Thymidylate synthase inhibition by an oral regimen consisting of tegafur-uracil (UFT) and low-dose leucovorin for patients with gastric cancer. Cancer Chemother Pharmacol 1996; 38: 401–5
Pazdur R, Ho D, Covington W, et al. Comparative steady state pharmacokinetics of oral UFT versus protracted intravenous 5-fluorouracil. Proc Am Soc Clin Oncol 1996; 15: 474A
Pazdur R, Lassere Y, Diaz-Canton E. Phase I trial of uraciltegafur (UFT) plus oral leucovorin: 14-day schedule. Invest New Drugs 1997; 15: 123–8
Meropol N, Rustum Y, Petrelli N, et al. A phase I and pharmacokinetic study of oral uracil, ftorafur, and leucovorin in patients with advanced cancer. Cancer Chemother Pharmacol 1996; 37: 581–6
Muggia F, Wu X, Spicer D, et al. Phase I and pharmacokinetic study of oral UFT, a combination of the 5-fluorouracil prodrug tegafur and uracil. Clin Cancer Res 1996; 2: 1461–7
Saltz L, Leichman C, Young C, et al. A fixed-ratio combination of uracil and ftorafur (UFT) with low dose leucovorin. An active oral regimen for advanced colorectal cancer. Cancer 1995; 75: 782–5
Jaiyesimi I, Pazdur R, Rhodes V, et al. Phase I study of tegafur plus uracil coadministered with oral leucovorin. Proc Am Soc Clin Oncol 1994; 13: 164A
Priest D, Schmitz J, Bunni M, et al. Pharmacokinetics of leucovorin metabolites in human plasma as a function of dose administered orally and intravenously. J Natl Cancer Inst 1991; 83: 1806–12
Gonzalez-Baron M, Feliu J, de la Gandara I, et al. Efficacy of oral tegafur modulation by uracil and leucovorin in advanced colorectal cancer. Aphase II study. Eur J Cancer 1995; 31A: 2215–9
Sanchiz F, Milla A. Tegafur-uracil (UFT) plus folinic acid in advanced rectal cancer. Jpn J Clin Oncol 1994; 24: 322–6
Feliu J, Gonzalez-Baron M, Espinosa E, et al. Uracil and tegafur modulated with leucovorin: an effective regimen with low toxicity for the treatment of colorectal carcinoma in the elderly. Oncopaz Cooperative Group. Cancer 1997; 79: 1884–9
Krook J, Moertel C, Gunderson L, et al. Effective surgical adjuvant therapy for high risk rectal carcinoma. N Engl J Med 1991; 324: 709–15
Diaz-Canton E, Pazdur R. Preoperative combined oral UFT plus leucovorin and radiation therapy for rectal cancer. Oncology 1997; 11: 58–60
Murad A, de Andrade C, Delfino C, et al. Pharmacoeconomic evaluation of tegafur-uracil (UFT) vs fluorouracil for the management of colorectal cancer in Brazil and Argentina. Clin Drug Invest 1997; 13: 90–8
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hoff, P.M., Lassere, Y. & Pazdur, R. Tegafur/Uracil + Calcium Folinate in Colorectal Cancer. Drugs 58 (Suppl 3), 77–83 (1999). https://doi.org/10.2165/00003495-199958003-00011
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-199958003-00011