Summary
Synopsis
Octreotide is a somatostatin analogue: a long-acting release (LAR) formulation of octreotide is designed for once-monthly intramuscular administration. As with native somatostatin, octreotide LAR exerts potent inhibitory effects on the secretion of growth hormone and on various peptides of the gastroenteropancreatic endocrine system.
When patients with acromegaly who show a positive response to treatment with subcutaneous octreotide 300 to 600 µg/day are switched to octreotide LAR 20 or 30mg, the resulting decrease in growth hormone levels is stable and sustained.
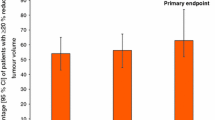
Reductions in growth hormone levels to <5 µg/L for about 4 weeks are seen in 86 to 100% of patients, to <2 to 2.5 µg/L in 39 to 75% and to <1 µg/L in 24 to 40%. Levels of insulin-like growth factor- 1 (IGF- 1) decrease in parallel and are often normalised with repeated drug treatment. There is no evidence of tachyphylaxis with long term therapy (up to 34 months). Treatment with octreotide LAR improves facial appearance and soft tissue thickening, and eliminates or reduces the incidence of symptoms such as headache, fatigue, arthralgia and excessive perspiration. Tumour shrinkage has been noted in some, but not all, patients receiving octreotide LAR, although this has not been widely evaluated in clinical studies.
Overall, octreotide LAR is well tolerated, and the mild to moderate gastrointestinal events experienced by up to 50% of patients are of short duration and often subside with continued drug administration. The incidence of gallbladder abnormalities (sediment, sludge, microlithiasis and gallstones) increases in patients receiving long term therapy with subcutaneous octreotide, although most patients remain asymptomatic. The incidence of gallbladder abnormalities in patients receiving octreotide LAR compares favourably with that during subcutaneous administration. Glycaemic control is not usually altered during octreotide LAR treatment.
In summary, octreotide continues to be the principal pharmacological option for most patients with acromegaly. Octreotide LAR offers the convenience of once-monthly administration compared with daily subcutaneous drug administration. In addition, the good efficacy and tolerability profile of octreotide LAR should enhance patient compliance and acceptability of octreotide therapy and contribute to an improvement in patient quality of life.
Pharmacodynamic Properties
The pharmacodynamic properties of the long-acting release (LAR) formulation of octreotide do not appear to differ qualitatively from those of the subcutaneously administered formulation. A single intramuscular dose of octreotide LAR 20 or 30mg consistently reduced growth hormone levels to less than 5 µg/L for about 4 weeks; this was similar to reductions achieved with daily subcutaneous administration of octreotide (300 or 600 jig/day). The extent of growth hormone suppression was similar between the 2 doses of octreotide LAR but tended to persist for longer in patients who received 30mg (up to 60 days) than in those treated with 20mg (up to 28 days). Reductions were also seen in insulin-like growth factor-1 (IGF-1) levels. In general, doses of octreotide LAR ≤10mg were not as effective as 20 or 30mg.
Treatment with octreotide LAR for up to 1 year was not associated with receptor down-regulation or drug tachyphylaxis, nor did octreotide antibodies develop in patients over this period.
Octreotide inhibits the secretion of various endocrine hormones (such as insulin, glucagon, gastric inhibitory peptide, secretin, gastrin, neurotensin and motilin); it also influences intestinal motility, blood flow to the gut, and carbohydrate, electrolyte and water balance. Drug-induced impairment of gallbladder and sphincter of Oddi motility, inhibition of bile secretion, and altered hepatic and biliary acid composition are thought to contribute to the increased incidence of biliary tract dysfunction and gallstones in patients who receive long term octreotide therapy (see Tolerability summary below).
Untreated acromegaly results in myocardial hypertrophy, which leads to left ventricular dysfunction and heart failure. Subcutaneous octreotide 300 to 1500 µg/day induced rapid and sustained improvement in cardiac indices which included a decrease in left ventricular mass, decrease in heart rate, improved systolic and diastolic function indices, increase in stroke volume (in patients with congestive heart failure) and an increase in exercise capacity. Improvement in cardiac indices has not been reported with octreotide LAR, but would also be expected with this formulation.
Pharmacokinetic Properties
Octreotide LAR comprises a biodegradable polymer matrix which releases octreotide in a biphasic manner. An initial peak in serum octreotide concentrations seen within 1 hour of administration in patients with acromegaly is due to drug release from the surface of the microspheres. This peak coincides with an 8- to 12-hour period of growth hormone suppression (level not specified) which is similar to that seen after subcutaneous drug administration. Serum octreotide concentrations decline within 12 hours and remain low until 7 days after the initial injection; they then increase in a dose-dependent manner and plateau at about day 14. The plateau concentration remains stable until day 35 to 60 and then steadily declines.
In patients with acromegaly treated with octreotide LAR 20 or 30mg, therapeutic octreotide concentrations (usually 1 to 3 µg/L) are reached during the plateau phase and suppression of growth hormone secretion is maximal (levels reduced to between 2 and 5 µg/L). Octreotide concentrations reach steady-state in patients with acromegaly after 3 intramuscular injections of octreotide LAR at 4-week intervals.
Octreotide distributes mainly to the plasma and is approximately 65% protein bound. In patients with acromegaly, the volume of distribution is about 18 to 30L. The total body clearance rate in healthy individuals is about 9.6 L/h but is increased to 18 L/h in patients with acromegaly and decreases to 4.5 L/h in patients with chronic renal failure. About 11 to 32% of the drug is eliminated unchanged in the urine.
Therapeutic Use
The therapeutic efficacy of octreotide in the management of patients with acromegaly is well established. Repeated intramuscular administration of octreotide LAR (usually 20 or 30mg every 28 days) for up to 34 months effectively suppressed growth hormone levels to <5 µg/L in 86 to 100% of patients who had previously responded to subcutaneous octreotide 3 times daily. Growth hormone levels were further reduced to <2 or 2.5 µg/L in 39 to 75% and to <1 µg/L in 24 to 40% of these patients (n = 8 to 101). Similarly, serum levels of IGF-1 were markedly reduced from baseline over the same treatment period and normalised in 64 to 88% of patients in 3 of 4 trials.
After 7 injections of octreotide LAR 20 to 40mg (administered once every 4 weeks), symptoms such as headache, fatigue, carpal tunnel syndrome, paraesthesia and excessive perspiration disappeared in 47 to 81% of patients (n = 37 to 66) in the largest clinical trial. Similarly, up to 60% of patients in smaller trials became asymptomatic after treatment for ≤19 months. Facial appearance, soft tissue thickening, memory and concentration also improved. Tumour shrinkage was noted in 29 to 72% of patients treated long term with octreotide LAR 20 to 40mg (n = 7 to 32) who had previously responded to daily subcutaneous octreotide, but it was not clear whether this correlated with suppression of growth hormone or improvement in symptoms.
Tolerability
Overall, the tolerability of octreotide LAR resembles that of the subcutaneously administered formulation, although prospective comparative data are not available. Gastrointestinal adverse events such as abdominal pain, flatulence, diarrhoea, constipation, steatorrhoea, nausea and vomiting predominate after 1 to 3 doses of intramuscular octreotide LAR 10 to 30mg and occur in up to 50% of patients. However, these experiences are mild to moderate in severity and usually persist for only 1 to 4 days. Mild or moderate pain at the injection site was reported in up to 45% of patients, while erythema and swelling were less common; these events were generally of short duration.
Gallstones develop in fewer than 2% of patients treated with subcutaneous octreotide for less than 1 month; stones or sludge occur in approximately 20 to 50% of those who receive longer term therapy but most patients remain asymptomatic. Surgical treatment or bile acid therapy may be required in the approximately 1% of patients who become symptomatic. Many of the clinical trials with octreotide LAR reported gallbladder abnormalities (sediment, sludge, microlithiasis, gallstones), but at a lower frequency (<15%) and no patient required therapeutic intervention.
Impaired glucose tolerance was not reported in any of the clinical trials with octreotide LAR, nor did treatment result in significant changes in vital signs, haematology or blood chemistry.
Dosage and Administration
Intramuscular octreotide LAR is indicated for the treatment of acromegaly in patients who are adequately controlled on subcutaneous administration of octreotide, or in whom surgery, radiotherapy or dopamine agonist therapy is inappropriate or ineffective, or until the full effect of radiotherapy is realised.
Patients who respond well to subcutaneous octreotide 300 to 600 µg/day may be switched to octreotide LAR 20mg administered intramuscularly at 4-week intervals for 3 months. If clinical symptoms, and growth hormone and IGF-1 levels are not controlled satisfactorily within this period the dose may be increased to 30mg. Conversely, the dose of octreotide LAR may be reduced to 10mg in patients in whom treatment for 3 months consistently results in growth hormone levels <1 µg/L, normal IGF-1 levels and disappearance of most reversible clinical signs of acromegaly.
No dosage adjustment is necessary in elderly patients (e65 years) or those with impaired renal function (not defined) or liver cirrhosis. Baseline and periodic ultrasound examinations are recommended to monitor for gallbladder abnormalities in patients treated with octreotide LAR.
Similar content being viewed by others
References
Lamberts SWJ, van der Lely A-J, de Herder WW, et al. Octreotide. N Engl J Med 1996 Jan 25; 334: 246–54
Daniels GH, Martin JB. Neuroendocrine regulation and diseases of the anterior pituitary and hypothalamus. In: Isselbacher KJ, Braunwald E, Wilson JD, et al., editors. Harrison’s principles of internal medicine. 13th ed. v. 2. New York: McGraw-Hill, Inc., 1994: 1891–918
Battershill PE, Clissold SP. Octreotide. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in conditions associated with excessive peptide secretion. Drugs 1989 Nov; 38: 658–702
Chanson P, Timsit J, Harris AG. Clinical pharmacokinetics of octreotide. Therapeutic applications in patients with pituitary tumours. Clin Pharmacokinet 1993 Nov; 25: 375–91
Flogstad AK, Halse J, Haldorsen T, et al. Sandostatin LAR in acromegalic patients: a dose-range study. J Clin Endocrinol Metab 1995 Dec; 80: 3601–7
Lancranjan I, Bruns C, Grass P, et al. Sandostatin LAR®: pharmacokinetics, pharmacodynamics, efficacy, and tolerability in acromegalic patients. Metabolism 1995 Jan; 44: 18–26
Lancranjan I, Bruns C, Grass P, et al. Sandostatin® LAR®: a promising therapeutic tool in the management of acromegalic patients. Metabolism 1996 Aug; 45(8) Suppl. 1: 67–71
Osman IA, Kendall-Taylor P. Use of a somatostatin analogue with prolonged action for treatment of acromegaly [abstract no. P162]. J Endocrinol 1994; 140 Suppl.
Priou A, Levesque G, Simonetta C, et al. Long acting sandostatine (Sandostatine LAR) in the treatment of acromegaly [in French]. Ann Endocrinol 1995; 56(3): 213–8
Shaw JAM, Hunter SJ, Wood PJ, et al. Comparison of subcutaneous and long-acting intramuscular octreotide in the treatment of acromegaly using different markers of GH activity [Poster P2-267]. 10th International Congress of Endocrinology; 1996 Jun 12–15; San Francisco, USA
Stewart PM, Kane KF, Stewart SE, et al. Depot long-acting somatostatin analog (Sandostatin LAR) is an effective treatment for acromegaly. J Clin Endocrinol Metab 1995 Nov; 80: 3267–72
Findling JW, Tyrrell JB. Anterior Pituitary Gland. In: Greenspan FS, editor. Basic and clinical endocrinology. 3rd ed. Norwalk: Appleton & Lange, 1991: 79–132
Jaffe CA, Barkan AL. Acromegaly: recognition and treatment. Drugs 1994 Mar; 47: 425–45
Grass P, Marbach P, Bruns C, et al. Sandostatin® LAR® (microencapsulated octreotide acetate) in acromegaly: pharmacokinetic and pharmacodynamic relationships. Metabolism 1996 Aug; 45(8) Suppl. 1: 27–30
Kaal A, Frystyk J, Skjaerbaek C, et al. Effects of intramuscular microsphere-encapsulated octreotide on serum growth hormone, insulin-like growth factors (IGFs), free IGFs, and IGF-binding proteins in acromegalic patients. Metabolism 1995 Jan; 44: 6–14
Bates AS, Evans AJ, Jones P, et al. Assessment of GH status in acromegaly using serum growth hormone, serum insulin-like growth factor-I and urinary growth hormone excretion. Clin Endocrinol 1995; 42(4): 417–23
Barkan AL, Beitins IZ, Kelch RP. Plasma insulin-like growth factor-I/somatomedin-C in acromegaly: correlation with the degree of growth hormone hypersecretion. J Clin Endocrinol Metab 1988 Jul; 67: 69–73
Vance ML, Harris AG. Long-term treatment of 189 acromegalic patients with the somatostatin analog octreotide. Results of the International Multicenter Acromegaly Study Group. Arch Intern Med 1991 Aug; 151: 1573–8
Arosio M, Macchelli S, Rossi CM, et al. Effects of treatment with octreotide in acromegalic patients — a multicenter Italian study. Eur J Endocrinol 1995 Oct; 133: 430–9
McKnight JA, McCance DR, Sheridan B, et al. Four years’ treatment of resistant acromegaly with octreotide. Eur J Endocrinol 1995 Apr; 132: 429–32
Newman CB, Melmed S, Snyder PJ, et al. Safety and efficacy of long term octreotide therapy of acromegaly: results of a multicenter trial in 103 patients-a clinical research center study. J Clin Endocrinol Metab 1995 Sep; 80: 2768–75
van der Lely AJ, Harris AG, Lamberts SW. The sensitivity of growth hormone secretion to medical treatment in acromegalic patients: influence of age and sex. Clin Endocrinol 1992 Aug; 37: 181–5
Ezzat S, Kontogeorgos G, Redelmeier DA, et al. In vivo responsiveness of morphological variants of growth hormone-producing pituitary adenomas to octreotide [see comments]. Eur J Endocrinol 1995 Dec; 133: 686–90
Reubi JC, Landolt AM. The growth hormone responses to octreotide in acromegaly correlate with adenoma somatostatin receptor status. J Clin Endocrinol Metab 1989 Apr; 68: 844–50
Bertherat J, Chanson P, Dewailly D, et al. Resistance to somatostatin (SRIH) analog therapy in acromegaly. Re-evaluation of the correlation between the SRIH receptor status of the pituitary tumor and the in vivo inhibition of GH secretion in response to SRIH analog. Horm Res 1992; 38(1–2): 94–9
Boanta C, Dumitrache C, Simonescu L, et al. Results recorded in 36 acromegalic patients treated long-term with Sandostatin® (SMS) LAR® [Poster P2-449]. 10th International Congress of Endocrinology; 1996 Jun 12–15; San Francisco, USA
Koop BL, Harris AG, Ezzat S. Effect of octreotide on glucose tolerance in acromegaly. Eur J Endocrinol 1994 Jun; 130: 581–6
Breidert M, Pinzer T, Wildbrett J, et al. Long-term effect of octreotide in acromegaly on insulin resistance. Horm Metab Res 1995 May; 27: 226–30
Ho KKY, Jenkins AB, Furier SM, et al. Impact of octreotide, a long-acting somatostatin analogue, on glucose tolerance and insulin sensitivity in acromegaly. Clin Endocrinol 1992 Mar; 36: 271–9
Sato K, Takamatsu K, Hashimoto K. Short-term effects of octreotide on glucose tolerance in patients with acromegaly. Endocr J 1995 Dec; 42: 739–45
James RA, Møller N, Chatterjee S, et al. Carbohydrate tolerance and serum lipids in acromegaly before and during treatment with high dose octreotide. Diabetic Med 1991 Jul; 8: 517–23
Pedroncelli A, Lancranjan I, Montini M, et al. Efficacy and safety of Sandostatin® LAR® during long-term treatment of acromegaly [abstract no. P2-447]. 10th International Congress of Endocrinology; 1996 Jun 12–13; San Francisco, USA
Cohen R, Chanson P, Bruckert E, et al. Effects of octreotide on lipid metabolism in acromegaly. Horm Metab Res 1992 Aug; 24: 397–400
Sacca L, Cittadini A, Fazio S. Growth hormone and the heart. Endocr Rev 1994 Oct; 15: 555–73
Lim MJ, Barkan AL, Buda AJ. Rapid reduction of left ventricular hypertrophy in acromegaly after suppression of growth hormone hypersecretion. Ann Intern Med 1992 Nov 1; 117: 719–26
Merola B, Cittadini A, Colao A, et al. Chronic treatment with the somatostatin analog octreotide improves cardiac abnormalities in acromegaly. J Clin Endocrinol Metab 1993 Sep; 77: 790–3
Pereira JL, Rodriguez-Puras MJ, Leal-Cerro A, et al. Acromegalic cardiomyopathy improves after treatment with increasing doses of octreotide. J Endocrinol Invest 1991 Jan; 14: 17–23
Tokgözoglu SL, Erbas T, Aytemir K, et al. Effects of octreotide on left ventricular mass in acromegaly. Am J Cardiol 1994 Nov 15; 74: 1072–4
Giustina A, Boni E, Romanelli G, et al. Cardiopulmonary performance during exercise in acromegaly, and the effects of acute suppression of growth hormone hypersecretion with octreotide. Am J Cardiol 1995 May 15; 75: 1042–7
Chanson P, Timsit J, Masquet C, et al. Cardiovascular effects of the somatostatin analog octreotide in acromegaly. Ann Intern Med 1990 Dec 15; 113: 921–5
Padayatty SJ, Perrins EJ, Belchetz PE. Octreotide treatment increases exercise capacity in patients with acromegaly. Eur J Endocrinol 1996 May; 134: 554–9
Dowling RH, Hussaini SH, Murphy GM, et al. Gallstones during octreotide therapy. Metabolism 1992 Sep; 41(9) Suppl 2: 22–33
Redfern JS, Fortuner II WJ. Octreotide-associated biliary tract dysfunction and gallstone formation: pathophysiology and management. Am J Gastroenterol 1995 Jul; 90: 1042–52
van Liessum PA, Swinkels LM, Pieters GF, et al. Lack of antibody formation during long-term subcutaneous treatment with the somatostatin analogue octreotide in acromegaly. Acta Endocrinol Copenh 1990 Mar; 122: 309–12
Ørskov H, Christensen SE, Weeke J, et al. Effects of antibodies against octreotide in two patients with acromegaly. Clin Endocrinol 1991 May; 34: 395–8
Musolino NR, Marino Jr R, Bronstein MD. Headache in acromegaly: dramatic improvement with the somatostatin analogue SMS 201–995. Clin J Pain 1990 Sep; 6: 243–5
Schmidt K, Althoff PH, Harris AG, et al. Analgesic effect of the somatostatin analogue octreotide in two acromegalic patients. A double-blind study with long-term follow-up. Pain 1993 May; 53: 223–7
Sheppard MC, Stewart PM. Use of long-acting somatostatin analogs in treating acromegaly. Endocrinologist 1995; 5(6): 456–9
Flogstad AK, Halse J, Bakke S, et al. Sandostatin LAR in acromegalic patients: long term treatment. J Clin Endocrinol Metab 1997; 82(1): 23–8
Sandoz Pharmaceuticals Ltd. Octreotide prescribing information
Hennessey JV, Jackson IMD. Clinical features and differential diagnosis of pituitary tumours with emphasis on acromegaly. Baillieres Clin Endocrinol Metab 1995; 9(2): 271–314
National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health. Acromegaly. Information for the public, patients, health educators, and health care providers (http://www.niddk.nih.gov/Acromegaly/Acromeg.html)
Krishna AY, Phillips LS. Management of acromegaly: a review. Am J Med Sci 1994 Dec; 308: 370–5
Davies PH, Stewart SE, Sheppard MC, et al. Continuing therapy with long acting octreotide (Sandostatin-LAR®) in the treatment of acromegaly [abstract P157]. J Endocrinol 1996; 148Suppl. 1
Ezzat S, Snyder PJ, Young WF, et al. Octreotide treatment of acromegaly. A randomized, multicenter study. Ann Intern Med 1992 Nov 1; 117: 711–8
Sandoz Pharmaceuticals Ltd. Octreotide LAR® prescribing information 57. Consensus statement: benefits versus risks of medical therapy for acromegaly. Acromegaly Therapy Consensus Development Panel. Am J Med 1994 Nov; 97: 468–73
Weekes LM, Ho KK, Seale JP. Treatment options in acromegaly: benefits and costs. PharmacoEconomics 1996 Nov; 10(5): 453–9
Fahlbusch R, Giovanelli M, Buchfelder M, et al. Advances in the medical and surgical treatment of pituitary adenomas: the role of long-acting somatostatin analogs. Participants of the “Conference on Medical and Surgical Treatment of Pituitary Adenomas” (Zurich, 5th October, 1991). J Endocrinol Invest 1993 Jun; 16: 449–60
Melmed S. Comprehensive management of acromegaly. Metab Clin Exp 1995; 44(1) Suppl.: 27–30
Jenkins D, O’Brien I, Johnson A, et al. The Birmingham pituitary database: auditing the outcome of the treatment of acromegaly. Clin Endocrinol 1995; 43: 517–22
Sheaves R, Jenkins P, Blackburn P, et al. Outcome of transsphenoidal surgery for acromegaly using strict criteria for surgical care. Clin Endocrinol 1996; 45: 407–13
Christensen SE, Weeke J, Ørskov H, et al. Long-term efficacy and tolerability of octreotide treatment in acromegaly. Metabolism 1992 Sep; 41Suppl. 2: 44–50
James RA, White MC, Chatterjee S, et al. A comparison of octreotide delivered by continuous subcutaneous infusion with intermittent injection in the treatment of acromegaly. Eur J Clin Invest 1992 Aug; 22: 554–61
Robbins RJ. Editorial: depot somatostatin analogs-a new front line therapy for acromegaly. J Clin Endocrinol Metab 1997; 82(1): 15–7
Bates AS, Van’t Hoff W, Jones JM, et al. An audit of outcome of treatment in acromegaly. Q J Med 1993; 86: 293–9
O’Halloran DJ, Shalet SM. Acromegaly: unravelling a complex disease. Growth Regul 1995 Sep; 5: 119–24
Morange I, De Boisvilliers F, Chanson P, et al. Slow release lanreotide treatment in acromegalic patients previously normalized by octreotide. J Clin Endocrinol Metab 1994 Jul; 79: 145–51
Caron P, Cogne M, Gusthiot-Joudet B, et al. Intramuscular injections of slow-release lanreotide (BIM 23014) in acromegalic patients previously treated with continuous subcutaneous infusion of octreotide (SMS 201–995). Eur J Endocrinol 1995 Mar; 132: 320–5
Giusti M, Gussoni G, Cuttica CM, et al. Effectiveness and tolerability of slow release lanreotide treatment in active acromegaly: six-month report on an Italian Multicenter study. J Clin Endocrinol Metab 1996; 81(6): 2089–97
Caron P, Morange-Ramos I, Cogne M, et al. Three year follow-up of acromegalic patients treated with intramuscular slow-release lanreotide. J Clin Endocrinol Metab 1997; 82(1): 18–22
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: D.R. Abernethy, Departments of Medicine and Pharmacology, Georgetown University Medical Center, Washington, D.C., USA; M. Arosio, Institute of Endocrine Sciences, University of Milan, Milan, Italy; S. Ezzat, Department of Medicine, The Wellesley Hospital, Toronto, Ontario, Canada; S.K. Fløgstad, Section of Endocrinology, Medical Department B, Rikshospitalet, Oslo, Norway; A. Giustina, Department of Endocrinology, University of Brescia, Brescia, Italy; C. Jaffe, Division of Endocrinology and Metabolism, University of Michigan Medical Center, Ann Arbor, Michigan, USA; H. Ørskov, Institute of Experimental Clinical Research, University Hospital, Aarhus, Denmark; P.M. Stewart, Department of Medicine, Queen Elizabeth Hospital, Edgbaston, Birmingham, England; L. Weekes, NSW Therapeutic Assessment Group Incorporated, Darlinghurst, New South Wales, Australia.
Rights and permissions
About this article
Cite this article
Gillis, J.C., Noble, S. & Goa, K.L. Octreotide Long-Acting Release (LAR). Drugs 53, 681–699 (1997). https://doi.org/10.2165/00003495-199753040-00009
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-199753040-00009