Abstract
Synopsis
Reteplase (BM 06.022; r-PA) is a recombinant peptide which consists of the kringle 2 and protease domains of human tissue-type plasminogen activator. It has been developed as a thrombolytic treatment for acute myocardial infarction (AMI). The half-life of reteplase allows administration as a double-bolus injection (second injection given 30 minutes after the first) rather than by the prolonged and, in some cases, more complex intravenous infusion regimens that are required for most other thrombolytic agents.
Reteplase produced rapid and effective coronary artery thrombolysis in a number of dose-finding and comparative studies. Double-bolus administration of reteplase 10U + JOU produced significantly higher coronary artery patency rates than accelerated alteplase (WOmg as a 1.5-hour infusion) in patients with AMI in the RAPID-II study. The 10U + 10U reteplase regimen produced a 35-day survival rate at least equivalent to that seen with a 1-hour infusion of streptokinase 1.5 million units in 5986 patients in the INJECT study, which was designed to demonstrate equivalence between treatments.
As with other thrombolytics, bleeding was the most common adverse event seen in reteplase recipients. No significant differences in the overall risk of haemorrhage were observed between reteplase and either accelerated alteplase or standard streptokinase treatment in clinical trials. The risk of stroke in reteplase recipients appears to be similar to that for other thrombolytic agents [1.2% incidence in 3288 patients treated with reteplase 10U + 10U in clinical trials (0.76% for haemorrhagic stroke)], although accurate statistical assessment of the relative risk is not possible for the data available to date.
Thus, reteplase is an effective thrombolytic agent which can be administered as a double-bolus injection regimen rather than as a prolonged infusion. Together with acquisition cost and general pharmacoeconomic data (which are not yet available), the results of GUSTO-III (a trial comparing double-bolus reteplase with accelerated alteplase in 15 000 patients) will have a major influence on the pattern of use of reteplase. In the meantime, data from the available clinical trials suggest that reteplase is a fast-acting and effective thrombolytic treatment for patients with AMI.
Pharmacodynamic Properties
Reteplase (BM 06.022; r-PA) is a 39.6kD, single-chain, nonglycosylated peptide which consists of amino acids 1 to 3 and 176 to 527 of native tissue-type plasminogen activator (t-PA). It contains the kringle 2 and protease domains of native t-PA, but lacks the kringle 1, finger and epidermal growth factor domains. Reteplase is produced by recombinant DNA technology in Escherichia coli and requires in vitro folding to become active.
Reteplase has lower in vitro affinity for fibrin than alteplase, probably because of the absence of the fibrin-binding finger domain which is part of native t-PA. It was less potent than alteplase for 50% clot lysis in vitro, but produced a similar maximum level of clot lysis in platelet-poor human plasma. Reteplase was a more potent thrombolytic than alteplase in canine models of coronary artery thrombosis. In addition, reteplase produced reperfusion significantly faster than alteplase, anistreplase, streptokinase or urokinase in canine models. In patients with acute myocardial infarction (AMI), reteplase reduced plasminogen levels to between 35 and 52% of baseline values, fibrinogen to between 36 and 61% of baseline and α2-antiplasmin to between 16 and 31% of baseline within 4 hours of treatment.
Pharmacokinetjc Properties
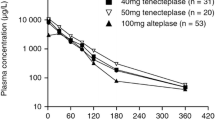
Maximum plasma concentrations of 2000 IU/ml (functional activity assay) and 4200 μg/L (detection of the reteplase antigen with a murine monoclonal antibody) were recorded after double-bolus intravenous administration of reteplase 10U + 10U (the recommended dose for clinical use) in patients with AMI. Data from healthy volunteers suggest that not all of the reteplase antigen in plasma is active. The initial half-life (t1/2α) of reteplase activity or antigen ranged from about 11 to 19 minutes in healthy volunteers or patients with AMI, compared with a typical t1/2α for alteplase of 5 minutes in other studies. Data from animal studies suggest that reteplase is cleared by both the liver and kidneys, but the latter route appears to be dominant. Inactivation of reteplase by components of blood or plasma also accounts for some of its elimination.
Clinical Efficacy
Reteplase produced rapid and effective coronary artery thrombolysis (as assessed by angiography) in dose-ranging and comparative studies in patients with AMI. In the RAPID-I study, double-bolus injection of reteplase 10U + 10U was significantly more effective than a 3-hour alteplase infusion, based on the rates of TIMI grade 3 (complete) patency at 60 and 90 minutes. There was no significant difference between the 2 treatments for the combined TIMI 2 and TIMI 3 (TIMI 2/3) flow rate at these times. Reteplase 10U + 10U produced more rapid thrombolysis than accelerated alteplase (100mg as a 1.5-hour infusion) in the RAPID-II study. Coronary artery patency rates were significantly greater with reteplase than with accelerated alteplase after 60 minutes [81.8 vs 66.1% (TIMI 2/3) and 51.2 vs 37.4% (TIMI 3)] and 90 minutes [83.4 vs 73.3% and 59.9 vs 45.2%]. The incidence of angiographically assessed reocclusion during hospitalisation was similar in reteplase (9.0%) and alteplase (7.0%) recipients.
Patients with AMI who received reteplase 10U + 10U had a 35-day survival rate at least equivalent to that seen with a 1-hour infusion of streptokinase 1.5 million units in the INJECT study (n = 5986). This multicentre trial was designed to show that the mortality rate for reteplase was not more than 1% greater than that for streptokinase; it was not sufficiently large to demonstrate superiority of one drug over the other. The 35-day mortality rate was 9.02% with reteplase and 9.53% with streptokinase, a difference of -0.51% (95% confidence interval -1.98 to 0.96%). Cardiogenic shock, heart failure, hypotension and atrial fibrillation occurred significantly less often in the reteplase group than in the streptokinase group.
Tolerability
Bleeding is the most common adverse event in reteplase recipients; about 21% of 3805 patients treated in clinical trials (3296 of whom had received reteplase 10U + 10U as a double bolus) experienced some type of bleeding. As with other thrombolytics, intracranial haemorrhage and haemorrhagic stroke are the most serious adverse events associated with reteplase. Stroke occurred in 1.2% of 3288 patients who received reteplase 10U + 10U in the RAPID-I, RAPID-II or INJECT studies (0.76% for haemorrhagic stroke). Bleeding events (of any sort) occurred in about 15 % of patients receiving either reteplase 10U + 10U or a 1 -hour infusion of streptokinase 1.5 million units in the INJECT trial. There were no significant differences between groups in the incidence of stroke (1.23 vs 1.00%) or haemorrhagic stroke (0.77 vs 0.37%). Similarly, no significant differences in any bleeding events were observed between reteplase 10U + 10U and alteplase given either as a 3-hour or a 1.5-hour infusion in RAPID-I or RAPID-II. However, it should be noted that the studies reported to date were not sufficiently large to allow accurate statistical analysis of differences in stroke rates. Data from 2400 patients treated with reteplase indicate that the drug is not antigenic.
Dosage and Administration
Reteplase treatment should be initiated as soon as possible after the onset of symptoms of AMI. The recommended dose is 2 separate 10U bolus injections, the second of which is given 30 minutes after the first. Reteplase recipients should also receive heparin and aspirin. Reteplase is currently recommended for coronary artery clot lysis, reduction of mortality and congestive heart failure and improvement in ventricular function in patients with AMI.
Similar content being viewed by others
References
Boehringer Mannheim (Mannheim). Summary of registration data package for Rapilysin™ (reteplase). June 15, 1995. (Data on file)
Boehringer Mannheim (Mannheim). Reteplase. Product Monograph. 1996. (Data on file)
Collen D, Lijnen HR, Todd PA, et al. Tissue-type plasminogen activator: a review of its pharmacology and therapeutic use as a thrombolytic agent. Drugs 1989; 38(3): 346–88
Granger CB, Califf RM, Topol EJ. Thrombolytic therapy for acute myocardial infarction: a review. Drugs 1992 Sep; 44: 293–325
Camiolo SM, Thorsen S, Astrup T. Fibrinogenolysis and fibrinolysis with tissue plasminogen activator, urokinase, streptokinase-activated human globulin, and plasmin. Proc Soc Exp Biol Med 1971; 138: 277–80
Hoylaerts M, Rijken DC, Lijnen HR, et al. Kinetics of the activation of plasminogen by human tissue plasminogen activator: role of fibrin. J Biol Chem 1982; 257(6): 2912–9
Topol EJ. Thrombolytic Intervention. In: Topol EJ, editor. Textbook of Interventional Cardiology. Philadelphia: W.B. Saunders Co., 1994: 68–111
Gillis JC, Wagstaff AJ, Goa KL. Alteplase: a reappraisal of its pharmacological properties and therapeutic use in acute myocardial infarction. Drugs 1995; 50(1): 102–36
Pennica D, Holmes WE, Kohr WJ, et al. Cloning and expression of human tissue-type plasminogen activator cDNA in E. coli. Nature 1983; 301: 214–21
Kohnert U, Rudolph R, Verheijen JH, et al. Biochemical properties of the kringle 2 and protease domains are maintained in the refolded t-PA deletion variant BM 06.022. Protein Eng 1992 Jan; 5: 93–100
Martin U, Bader R, Bohm E, et al. BM 06.022: a novel recombinant plasminogen activator. Cardiovasc Drug Rev 1993; 11(3): 299–311
Genetic engineering in cardiology. Br J Cardiol 1996 Apr: 89-93
Sturzebecher J, Neumann U, Kohnert U, et al. Mapping of the catalytic site of CHO-t-PA and the t-PA variant BM 06.022 by synthetic inhibitors and substrates. Protein Sci 1992 Aug; 1: 1007–13
Kohnert U, Horsch B, Fischer S. A variant of tissue plasminogen activator (t-PA) comprised of the kringle 2 and the protease domain shows a significant difference in the in vitro rate of plasmin formation as compared to the recombinant human t-PA from transformed Chinese hamster ovary cells. Fibrinolysis 1993 Nov; 7: 365–72
Martin U, Sponer G, Strein K. Differential fibrinolytic properties of the recombinant plasminogen activator BM 06.022 in human plasma and blood clot systems in vitro. Blood Coagul Fibrinolysis 1993 Apr; 4: 235–42
Martin U, Fischer S, Kohnert U, et al. Coronary thrombolytic properties of a novel recombinant plasminogen activator (BM 06.022) in a canine model. J Cardiovasc Pharmacol 1991 Jul; 18: 111–9
Martin U, Sponer G, Konig R, et al. Double bolus administration of the novel recombinant plasminogen activator BM-06.022 improves coronary blood flow after reperfusion in a canine model of coronary thrombosis. Blood Coagul Fibrinolysis 1992 Apr; 3: 139–47
Martin U, Sponer G, Strein K. Evaluation of thrombolytic and systemic effects of the novel recombinant plasminogen activator BM 06.022 compared with alteplase, anistreplase, streptokinase and urokinase in a canine model of coronary artery thrombosis. J Am Coll Cardiol 1992 Feb; 19: 433–40
Martin U, Fischer S, Sponer G. Influence of heparin and systemic lysis on coronary blood flow after reperfusion induced by the novel recombinant plasminogen activator BM 06.022 in a canine model of coronary thrombosis. J Am Coll Cardiol 1993 Sep; 22: 914–20
Martin U, Dalchau H, Sponer G. Effects of the novel recombinant plasminogen activator BM 06.022 on platelets and bleeding time in rabbits. Platelets 1992; 3(5): 247–53
Martin U, von Mollendorff E, Akpan W, et al. Pharmacokinetic and hemostatic properties of the recombinant plasminogen activator BM 06.022 in healthy volunteers. Thromb Haemost 1991 Nov; 66: 569–74
Martin U, von Mollendorff E, Akpan W, et al. Dose-ranging study of the novel recombinant plasminogen activator BM 06.022 in healthy volunteers. Clin Pharmacol Ther 1991 Oct; 50: 429–36
Seifried E, Muller MM, Martin U, et al. Bolus application of a novel recombinant plasminogen activator in acute myocardial infarction patients: pharmacokinetics and effects on the hemostatic system. Ann N Y Acad Sci 1992 Dec 4; 667: 417–20
Neuhaus K-L, von Essen R, Vogt A, et al. Dose finding with a novel recombinant plasminogen activator (BM 06.022) in patients with acute myocardial infarction: results of the German Recombinant Plasminogen Activator Study. J Am Coll Cardiol 1994 Jul; 24: 55–60
Seifried E, Muller M, Ziesche S, et al. Dose-ranging multicenter study (GRECO) of a novel recombinant plasminogen activator: influence on the hemostatic system [abstract]. Arterioscler Thromb 1991; 11(5): 1560a
Tebbe U, Von ER, Smolarz A, et al. Open, noncontrolled dosefinding study with a novel recombinant plasminogen activator (BM 06.022) given as a double bolus in patients with acute myocardial infarction. Am J Cardiol 1993 Sep 1; 72: 518–24
Grünewald M, Ellbrück D, Mohren M, et al. Single vs double bolus thrombolysis with the recombinant plasminogen activator BM 06.022 in patients with acute myocardial infarction-pharmacokinetics and hemostatic changes [abstract]. Thromb Haemost 1995 Jun; 73: 1328
Müller M, Haerer W, Ellbrück D, et al. Pharmacokinetics and effects on the hemostatic system of bolus application of a novel recombinant plasminogen activator in AMI patients [abstract no. 63]. Fibrinolysis 1992; 6 Suppl. 2: 26
Martin U, Fischer S, Kohnert U, et al. Pharmacokinetic properties of an Escherichia-coli-produced recombinant plasminogen activator (BM 06.022) in rabbits. Thromb Res 1991 May 1; 62: 137–46
Martin U, Kohler J, Sponer G, et al. Pharmacokinetics of the novel recombinant plasminogen activator BM 06.022 in rats, dogs, and non-human primates. Fibrinolysis 1992 Jan; 6: 39–43
Seifried E, Tanswell P, Rijken DC, et al. Pharmacokinetics of antigen and activity of recombinant tissue-type plasminogen activator after infusion in healthy volunteers. Arzneimittel Forschung 1988; 38 (Pt I) (3): 418–22
Kuiper J, van de Bilt H, Martin U, et al. Uptake, internalization and degradation of the novel plasminogen activator reteplase (BM 06.022) in the rat. Thromb Haemost 1995 Dec; 74: 1501–10
Martin U, Sponer G, Strein K. Influence of hepatic and renal failure on pharmacokinetic properties of the novel recombinant plasminogen activator BM 06.022 in rats. Drug Metab Dispos 1993 Mar–Apr; 21: 236–41
Martin U, Doerge L, Stegmeier K, et al. Influence of the degree of renal dysfunction on the pharmacokinetic properties of the novel recombinant plasminogen activator reteplase in rats. Drug Metab Dispos 1996 Mar; 24: 288–92
Rijken DC, Groeneveld E, Barrett-Bergshoeff MM. In vitro stability of a tissue-type plasminogen activator mutant, BM 06.022, in human plasma. Thromb Haemost 1994 Dec; 72: 906–11
International Joint Efficacy Comparison of Thrombolytics. Randomised, double-blind comparison of reteplase double-bolus administration with streptokinase in acute myocardial infarction (INJECT): trial to investigate equivalence. Lancet 1995 Aug 5; 346: 329–36
Smalling RW, Bode C, Kalbfleisch J. More rapid, complete, and stable coronary thrombolysis with bolus administration of reteplase compared with alteplase infusion in acute myocardial infarction. Circulation 1995 Jun 1; 91: 2725–32
Bode C, Smalling RW, Berg G, et al. Randomized comparison of coronary thrombolysis achieved with double bolus reteplase (r-PA) and front-loaded, ‘accelerated’ alteplase (rt-PA) in patients with acute myocardial infarction. Circulation 1996 Sep 1; 94(5): 891–8
Schroder R, Wegscheider K, Schroder K, et al. Extent of early ST segment elevation resolution: a strong predictor of outcome in patients with acute myocardial infarction and a sensitive measure to compare thrombolytic regimens. A substudy of the International Joint Efficacy Comparison of Thrombolytics (INJECT) trial. J Am Coll Cardiol 1995 Dec; 26(7): 1657–64
Ludlam CA, Bennett B, Fox KAA, et al. Guidelines for the use of thrombolytic therapy. Blood Coagul Fibrinolysis 1995; 6: 273–85
Cairns J, Armstrong P, Belenkie I, et al. Canadian Consensus Conference on Coronary Thrombolysis — 1994 recommendations. Can J Cardiol 1994 Jun; 10: 522–9
Cairns JA, Fuster V, Gore J, et al. Coronary thrombolysis. Chest 1995; 108(4 Suppl.): 401S–23S
Reteplase recommended for US approval. Scrip 1996; No. 2127/28 May 10/14: 23
Boehringer Mannheim reteplase label should avoid comparisons to Activase, FDA Advisory cmte. says; bleeding complications similar to streptokinase. FDC Pink 1996 May 6; 58: 3
Becker RC, Corrao JM, Harrington R, et al. Recombinant tissue-type plasminogen activator: current concepts and guidelines for clinical use in acute myocardial infarction. Part I. Am Heart J 1991 Jan; 121: 220–44
Machecourt J, Dumoulins J, Calop J, et al. Cost effectiveness of thrombolytic treatment for myocardial infarction: comparison of anistreplase, alteplase and streptokinase in 270 patients treated within 4 hours. Eur Heart J 1993; 14: 75–83
Braunwald E. The open-artery theory is alive and well — again. N Engl J Med 1993; 329(22): 1650–2
Simes RJ, Topol EJ, Holmes DR, et al. Link between the angiographic substudy and mortality outcomes in a large randomized trial of myocardial reperfusion: importance of early and complete infarct artery reperfusion. Circulation 1995; 91: 1923–8
The GUSTO Investigators. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med 1993; 329(10): 673–82
Mark DB, Hlatky MA, Califf RM, et al. Cost effectiveness of thrombolytic therapy with tissue plasminogen activator as compared with streptokinase for acute myocardial infarction [published erratum appears in N Engl J Med 1995; 333: 267]. N Engl J Med 1995 May 25; 332: 1418–24
Verstraete M, Lijnen HR, Collen D. Thrombolytic agents in development. Drugs 1995; 50(1): 29–42
Gruppo Italiano per lo Studio della Streptochinasi nell’Infarto miocardico (GISSI). Effectiveness of intravenous thrombo lytic treatment in acute myocardial infarction. Lancet 1986; I: 397–402
The European Myocardial Infarction Project Group. Prehospital thrombolytic therapy in patients with suspected acute myocardial infarction. N Engl J Med 1993; 329: 383–9
Fibrinolytic Therapy Trialists’ (FTT) Collaborative Group. Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients. Lancet 1994; 343: 311–22
The GUSTO Angiographic Investigators. The effects of tissue plasminogen activator, streptokinase, or both on coronary-artery patency, ventricular function, and survival after acute myocardial infarction. N Engl J Med 1993; 329(22): 1615–22
Author information
Authors and Affiliations
Additional information
Various sections of the manuscript reviewed by: C. Bode, Medizinischen Klinik III (Kardiologie), Universitat Heidelberg, Heidelberg, Germany; J.A. Cairns, Department of Medicine, McMaster University, Hamilton, Ontario, Canada; M. Ohman, Duke University Medical Center, Duke Clinical Research Institute, Durham, North Carolina, USA; D.C. Rijken, TNO-PG, Gaubius Laboratory, Leiden, The Netherlands; E. Seifried, Institut fur Transfusionsmedizin und Immunhämatologie, Frankfurt, Germany; M. Silver, The Cleveland Clinic Foundation, Cleveland, Ohio, USA; R.W. Smalling, Division of Cardiology, University of Texas Medical School at Houston, Houston, Texas, USA; E.J. Topol, Department of Cardiology, The Cleveland Clinic Foundation, Cleveland, Ohio, USA; M. Verstraete, Center for Molecular and Vascular Biology, Katholieke Universiteit Leuven, Leuven, Belgium; C.F.M. Weston, Pinderfields Hospital, Wakefield, England; H. White, Cardiology Department, Greenlane Hospital, Auckland, New Zealand.
Rights and permissions
About this article
Cite this article
Noble, S., McTavish, D. Reteplase. Drugs 52, 589–605 (1996). https://doi.org/10.2165/00003495-199652040-00012
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-199652040-00012