Summary
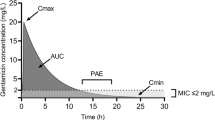
Despite the availability of newer and safer antibacterials, aminoglycosides continue to play a major role in the management of infections in hospitalised patients. The concept of single daily dose (SDD) regimens was introduced many years ago and is now receiving much attention as an alternative regimen for this class of drugs. To evaluate the rationale and clinical support for SDD schemes, we conducted a ‘MEDLINE’ search to locate relevant preclinical and clinical literature pertaining to this issue. The results of animal model and noncomparative clinical data tended to be variable and inconclusive. We were able to identify 28 prospective comparative clinical trials; however, only one was randomised, double-blind and of sufficient sample size to detect differences in efficacy between treatment arms, should any exist. Despite these flaws, our review suggests that SDD schemes appear to be no more efficacious and no less toxic, but may be less costly, than traditional multiple daily dose schemes. We also assessed the predicted disposition of tobramycin/gentamicin in 415 patients with known pharmacokinetic parameters. With doses of 7 mg/kg at intervals of between 24 and 60 hours (depending upon renal function), the maximum serum concentration at steady-state (Css max) varied from 8.5 to 55.6 mg/L, while the Css min was <2.0 mg/L in the majority of patients. Mid-interval serum aminoglycoside concentrations were <0.5 mg/L in up to 23% of patients, suggesting possible underdosage in certain patients with this scheme. More conclusive clinical evidence is necessary before SDD schemes should be adopted as standard clinical practice. Empirical weight-based dosage schemes appear to yield widely variable serum aminoglycoside concentrations which could be considered therapeutically inadequate or toxic.
Similar content being viewed by others
References
Waksman SA, Bugie E, Scheatz A. Isolation of antibiotic substances from soil microorganisms, with special reference to streptothricin and streptomycin. Proc Staff Meet May Clin 1944; 19: 537–48
Siegenthaler WE, Bonetti A, Luthy R. Aminoglycoside antibiotics in infectious diseases. An overview. Am J Med 1986; 80 Suppl. 6B: 2–14
Neu HC. Antibiotics in the second half of the 1980s. Areas of future development and the effect of new agents on aminoglycoside use. Am J Med 1986; 80 Suppl. 6B: 195–203
Bates RD, Nahata MC. Once-daily administration of aminoglycosides. Ann Pharmacother 1994; 28: 757–66
Gilbert DN. Aminoglycosides. In: Mandell GL, Douglas RG, Bennett JE, editors. Principles an practice of infectious diseases. New York: Churchill Livingstone, 1995: 279–306
Lyon MD, Smith KR, Saag MS, et al. In vitro activity of piperacillin, ticarcillin, and mezlocillin alone and in combination with aminoglycoside against Pseudomonas aeruginosa. Antimicrob Agents Chemother 1986; 30: 25–30
Johnson DE, Thompson B, Calia FM. Comparative activities of piperacillin, ceftazidime, and amikacin, alone and in all possible combinations, against experimental Pseudomonas aeruginosa infection in neutropenic rats. Antimicrob Agents Chemother 1985; 28: 735–9
Cunha BA. Aminoglycosides: current role in antimicrobial therapy. Pharmacotherapy 1988; 8: 334–50
Barriere SL. Aminoglycosides: a reassessment of their therapeutic role. Clin Pharm 1988; 7: 385–90
Gutensohn A, Bunz D, Frighetto L, et al. Outcome of a ceftriaxone/cefotaxime interchange programme in a major teaching hospital. Chemotherapy 1991; 37 Suppl. 3: 15–21
Labovitz E, Levison ME, Kaye D. Single-dose daily gentamicin therapy in urinary tract infection. Antimicrob Agents Chemother 1974; 6(4): 465–70
Klastersky J, Prevost JM, Meunier-Carpentier F, et al. Comparative trial of single-dose versus twice-daily sisomicin in bacteriuric patients. J Clin Pharmacol 1977; 17(8-9): 520–8
Cohen B, Saginur R, Clecner R, et al. Double-blind comparative trial of once- versus twice-daily netilmicin therapy in severe acute urinary tract infections. Curr Ther Res 1985; 38(6): 880–4
Van der Auwera P, Meunier F, Ibrahim S, et al. Pharmacodynamic parameters and toxicity of netilmicin (6 milligram/kilogram/day) given once daily or in three divided doses to cancer patients with urinary tract infection. Antimicrob Agents Chemother 1991; 35(4): 640–7
Shankar A, Sharma SD. Gentamicin as once-daily dose therapy in recurrent urinary tract infections in children. Curr Ther Res 1987; 41(5): 599–603
Angelov A, Barrientos G, Gutensohn G, et al. Die tagliche einmaldosis von gentamicin bei der behandlung von harnwegsinfektionen. Munch Med Wochenschr 1980; 122(6): 212–4
Vigano A, Principi N, Brivio L, et al. Comparison of 5 milligrams of netilmicin per kilogram of body weight once daily versus 2 milligrams per kilogram thrice daily for treatment of gram-negative pyelonephritis in children. Antimicrob Agents Chemother 1992; 36(7): 1499–503
Mendes da Costa P, Kaufman L. Amikacin once daily plus metronidazole versus amikacin twice daily plus metronidazole in colorectal surgery. Hepatogastroenterology 1992; 39: 350–4
de Vries PJ, Verkooyen RP, Leguit P, et al. Prospective randomized study of once-daily versus thrice-daily netilmicin regimens in patients with intra-abdominal infections. Eur J Clin Microbiol Infect Dis 1990; 9(3): 161–8
Fan ST, Lan WY, Teoh-Chan CH, et al. Once daily administration of netilmicin compared with thrice daily, both in combination with metronidazole, in gangrenous and perforated appendicitis. J Antimicrob Chemother 1988; 22: 69–74
Hollender LF, Bahnini J, DeManzini N, et al. A multicentric study of netilmicin once daily versus thrice daily in patients with appendicitis and other intra-abdominal infections. J Antimicrob Chemother 1989; 23: 773–83
Tulkens PM. Pharmacokinetic and toxicological evaluation of a once-daily regimen versus conventional schedules of netilmicin and amikacin. J Antimicrob Chemother 1991; 27 Suppl. C: 49–61
Heininger U, Bowing B, Stehr K, et al. Aminoglycoside bei patienten mit mukoviszidose und pulmonaler exazerbation: vergleich von einmal-und dreimalgabe. Klin Padiatr 1993; 205: 18–22
Mauracher EH, Lau WY, Kartowisastro H, et al. Comparison of once-daily and thrice-daily netilmicin regimens in serious systemic infections: a multicenter study in six Asian countries. Clin Ther 1989; 11(5): 604–13
Sturm AW. Netilmicin in the treatment of gram-negative bacteremia: single daily versus multiple daily dosage. J Infect Dis 1989; 159(5): 931–7
Nordstrom L, Ringberg H, Cronberg S, et al. Does administration of an aminoglycoside in a single daily dose affect its efficacy and toxicity? J Antimicrob Chemother 1990; 25: 159–73
Giamarellou H, Yiallouros K, Petrikkos G, et al. Comparative kinetics and efficacy of amikacin administered once or twice daily in the treatment of systemic gram-negative infections. J Antimicrob Chemother 1991; 27 Suppl. C: 73–9
TerBraak EW, de Vries PJ, Bouter KP, et al. Once-daily dosing regimen for aminoglycoside plus β-lactam combination therapy of serious bacterial infections: comparative trial with netilmicin plus ceftriaxone. Am J Med 1990; 89: 58–66
Maller R, Ahrne H, Holmen C, et al. Once- versus twice-daily amikacin regimen: efficacy and safety in systemic gram-negative infections. J Antimicrob Chemother 1993; 31: 939–48
Prins JM, Buller HR, Kuijper EJ, et al. Once versus thrice daily gentamicin in patients with serious infections. Lancet 1993; 341: 335–9
Vanhaeverbeek M, Siska G, Douchamps J, et al. Comparison of the efficacy and safety of amikacin once or twice-a-day in the treatment of severe gram-negative infections in the elderly. Int J Clin Pharmacol Ther Toxicol 1993; 31: 153–6
Marik PE, Lipman J, Kobilski S, et al. A prospective randomized study comparing once- versus twice-daily amikacin dosing in critically ill adult and paediatric patients. J Antimicrob Chemother 1991; 28: 753–64
Raz R, Adawi M, Romano S. Intravenous administration of gentamicin once daily versus thrice daily in adults. Eur J Clin Microbiol Infect Dis 1995; 14: 88–91
Hansen M, Achen F, Carstensen C, et al. Once- versus thrice-daily dosing of netilmicin in febrile immunocompromized patients: a randomized, controlled study of efficacy and safety. J Drug Dev 1988; 1 Suppl. 3: 119–24
Rozdzinski E, Kern WV, Reichle A, et al. Once- versus thrice-daily dosing of netilmicin in combination with β-lactam antibiotics as empirical therapy for febrile neutropenic patients. J Antimicrob Chemother 1993; 31: 585–98
Gibson J, Johnson L, Snowdon L, et al. Single daily ceftriaxone and tobramycin in the empirical management of febrile neutropenic patients: a randomized trial. Int J Hematol 1993; 58: 63–72
EORTC The International Antimicrobial Therapy Cooperative Group of the European Organization for Research and Treatment of Cancer. Efficacy and toxicity of single daily doses of amikacin and ceftriaxone versus multiple daily doses of amikacin and ceftazidime for infection in patients with cancer and granulocytopenia. Ann Intern Med 1993; 119: 584–93
Leoni F, Ciolli S, Pascarella A, et al. Ceftriaxone plus conventional or single-daily dose amikacin versus ceftazidime/amikacin as empiric therapy in febrile neutropenic patients. Chemotherapy 1993; 39: 147–52
Schumock GT, Raber SR, Crawford SY, et al. National survey of once-daily dosing of aminoglycoside antibiotics. Pharmacotherapy 1995; 15: 201–9
Nicolau DP, Freeman CD, Belliveau PP, et al. Experience with a once-daily aminoglycoside program administered to 2,184 adult patients. Antimicrob Agents Chemother 1995; 39: 650–5
Gilbert DN, Bennett WM. Use of antimicrobial agents in renal failure. Infect Dis Clin North Am 1989; 3: 517–31
Nicolau D, Quintiliani R, Nightingale C. Once-daily aminoglycosides. Conn Med 1992; 56(10): 561–3
McCormack JP, Jewesson PJ. A critical reevaluation of the ‘therapeutic range’ of aminoglycosides. Clin Infect Dis 1992; 14: 320–39
Begg EJ, Barclay ML, Duffull SB. A suggested approach to once-daily aminoglycoside dosing. Br J Clin Pharmacol 1995; 39: 605–9
Dudley MN, Zinner SH. Single daily dosing of amikacin in an in vitro model. J Antimicrob Chemother 1991; 27 Suppl. C: 15–19
Vogelman B, Gudmundsson S, Trunidge J, et al. In vivo postantibiotic effect in a thigh infection in neutropenic mice. J Infect Dis 1988; 157: 287–98
Vogelman BS, Craig WA. Postantibiotic effects. J Antimicrob Chemother 1985; 15 Suppl. A: 37–46
MacArthur RD, Lolans V, Zar FA, et al. Biphasic, concentration-dependent and rate limited, concentration-independent bacterial killing by an aminoglycoside antibiotic. J Infect Dis 1984; 150: 778–9
Daikos GL, Jackson GG, Lolans FT, et al. Adaptive resistance to aminoglycoside antibiotics from first-exposure down-regulation. J Infect Dis 1990; 162: 414–20
Daikos GL, Lolans VT, Jackson GG. First-exposure adaptive resistance to aminoglycoside antibiotics in vivo with meaning for optimal clinical use. Antimicrob Agents Chemother 1991; 35: 117–23
Barclay ML, Begg EJ, Chambers ST. Adaptive resistance following single doses of gentamicin in a dynamic in vitro model. Antimicrob Agents Chemother 1992; 36: 1951–7
Craig WA, Gudmundsson S. Postantibiotic effect. In: Lorian V, editor. Antibiotics in laboratory medicine. 3rd ed. Baltimore: The William & Wilkins Co. 1991: 403–31
Leggett JE, Fantin B, Ebert S, et al. Comparative antibiotic dose-effect relations at several dosing intervals in murine pneumonitis and thigh-infections models. J Infect Dis 1989; 159: 281–92
Moore RD, Lietman P, Smith CR. Clinical response to aminoglycoside therapy: importance of the ratio of peak concentration to minimal inhibitory concentration. J Infect Dis 1987; 155: 93–9
Bundtzen RW, Gerber AU, Cohn DL, et al. Post antibiotic suppression of bacterial growth. Rev Infect Dis 1981; 3: 28–37
Craig WA, Vogelman B. The postantibiotic effect. Ann Intern Med 1987; 106: 900–2
Fantin B, Ebert S, Leggett J, et al. Factors affecting duration of in-vivo postantibiotic effect for aminoglycosides against gram negative bacilli. J Antimicrob Chemother 1990; 27: 829–36
Isaksson B, Nilsson L, Moller, et al. Postantibiotic effect of aminoglycosides on gram negative bacteria evaluated by a new method. J Antimicrob Chemother 1988; 22: 23–33
Zhanel CG, Craig WA. Pharmacokinetic contributions to postantibiotic effects: focus on aminoglycosides. Clin Pharmacokinet 1994; 27(5): 377–92
Kapusnik JE, Hackbarth CJ, Chambers HF, et al. Single, large, daily dosing versus intermittent dosing of tobramycin for treating experimental Pseudomonas pneumonia. J Infect Dis 1988; 158: 7–12
Mackenzie FM, Gould IM. The post-antibiotic effect. J Antimicrob Chemother 1993; 32: 519–37
Karlowsky JA, Zhanel CG Davidson RJ, et al. In vitro postantibiotic effects following multiple exposures of cefotaxime, ciprofloxacin and gentamicin against Escherichia coli in pooled human cerebrospinal fluid and Mueller-Hinton broth. Antimicrob Agents Chemother 1993; 37: 1154–7
McGarth BJ, Marchbanks R, Gilbert D, et al. In vitro postantibiotic effect following recreated exposure to imipenem, temafloxacin and tobramycin. Antimicrob Agents Chemother 1993; 37: 1723–5
Gilbert DN. Once-daily aminoglycoside therapy. Antimicrob Agents Chemother 1991; 35(3): 399–405
Powell S, Thompson W, Luthe M, et al. Once-daily vs continuous aminoglycoside dosing: efficacy and toxicity in animal and clinical studies of gentamicin, netilmicin and tobramycin. J Infect Dis 1983; 147(5): 918–32
Mordend JJ, Quintiliani R, Nightingale CH. Combination antibiotic therapy: comparison of constant infusion and intermittent bolus dosing in an experimental animal model. J Antimicrob Chemother 1985; 15 Suppl. A: 313–21
Kapusnik JE, Sande MA. Novel approaches for the use of aminoglycosides: the value of experimental models. J Antimicrob Chemother 1986; 17 Suppl. A: 7–10
Potel A, Caillon J, Fantin B, et al. Impact of dosage schedule on the efficacy of gentamicin, tobramycin or amikacin in an experimental model of Serratia marcescens endocarditis: in vitro-in vivo correlation. Antimicrob Agents Chemother 1992; 36: 2403–7
Gerber AU, Craig WA, Brugger HP, et al. Impact of dosing intervals on activity of gentamicin and ticarcillin against Pseudomonas aeruginosa in granulocytopenic mice. J Infect Dis 1983; 147: 910–7
Pechere M, Letarte R, Pechere J-C. Efficacy of different dosing schedules of tobramycin for treating a murine Klebsiella pneumoniae bronchopneumonia. J Antimicrob Chemother 1987; 19: 487–91
Craig WA, Redington J, Ebert SC. Pharmacodynamics of amikacin in vitro and in mouse thigh and lung infections. J Antimicrob Chemother 1991; 27 Suppl. C: 29–40
Herscovici L, Grise G, Thauvin C, et al. Efficacy and safety of once daily versus intermittent dosing of tobramycin in rabbits with acute pyelonephritis. Scand J Infect Dis 1988; 20: 205–12
Wood CA, Norton DR, Kohlhepp SJ, et al. The influence of tobramycin dosing regimens on nephrotoxicity, ototoxicity and antibacterial efficacy in a rat model of subcutaneous abscess. J Infect Dis 1988; 158: 13–22
Weber M, Voirot P, Dopff C, et al. In vivo bactericidal activity of an oxacillin-netilmicin combination against Staphylococcus aureus. Influence of the rhythm of netilmicin administration. Pathol Biol 1988; 36: 389–93
Gerber AU, Kozak S, Segessenmann C, et al. Once-daily versus thrice-daily administration of netilmicin in combination therapy of Pseudomonas aeruginosa infection in a man-adapted neutropenic animal model. Eur J Clin Microbiol Infect Dis 1989; 8: 233–7
Gerber AU, Stritzko T, Segessenmann C, et al. Simulation human pharmacokinetic profiles in mice and impact on antimicrobial efficacy of netilmicin, ticarcillin and ceftazidime in the peritonitis-septicaemia model. Scand J Infect Dis Suppl 1990; 74: 195–203
Fantin B, Carbon C. Importance of aminoglycoside dosing regimen in the penicillin-netilmicin combination for treatment of Enterococcus faecalis-induced experimental endocarditis. Antimicrob Agents Chemother 1990; 34: 2387–91
Saleh-Mghir A, Cremieux A, Vallois J, et al. Optimal aminoglycoside dosing regimen for penicillin-tobramycin synergism in experimental Streptococcus adjacens endocarditis. Antimicrob Agents Chemother 1992; 36: 2403–7
Hatala M, Moravek J, Prat V, et al. Daily single dose gentamicin therapy in experimental pyelonephritis. Infection 1977; 5: 232–5
Chan GLC. Alternative dosing strategy for aminoglycosides: impact on efficacy, nephrotoxicity, and ototoxicity. DICP 1989; 23: 788–94
Barclay ML, Begg EJ, Hickling KG. What is the evidence for once-daily aminoglycoside therapy? Clin Pharmacokinet 1994; 27(1): 32–48
De Broe ME, Guilliano RA, Verpooten GA. Choice of drug and dosage regimen: two important risk factors for aminoglycoside nephrotoxicity. Am J Med 1986; 80 Suppl. 6B: 115–8
Silverblatt FJ, Kuehn C. Autoradiography of gentamicin uptake by the rat proximal tubule cell. Kidney Int 1979; 15: 335–45
Giuliano RA, Paulus GJ, Verpooten GA, et al. Recovery of cortical phospholipidosis and necrosis after acute gentamicin loading in rats. Kidney Int 1984; 26: 838–47
De Broe ME, Paulus GJ, Verpooten GA, et al. Early effects of gentamicin, tobramycin, and amikacin on the human kidney. Kidney Int 1984; 25: 643–52
Giuliano RA, Verpooten GA, DeBroe ME. The effect of dosing strategy on kidney cortical accumulation of aminoglycosides in rats. Am J Kidney Dis 1986; 8: 297–303
Giuliano RA, Verpooten GA, Verbist L, et al. In vivo uptake kinetics of aminoglycosides in the kidney cortex of rats. J Pharmacol Exp Ther 1986; 236: 470–5
Aronoff GR, Pottratz ST, Brier ME, et al. Aminoglycoside ac-cumulation kinetics in rat renal parenchyma. Antimicrob Agents Chemother 1983; 23: 74–8
Brier ME, Mayer PR, Brier RA, et al. Relationship between rat renal accumulation of gentamicin, tobramycin and netilmicin and their nephrotoxicities. Antimicrob- Agents Chemother 1985; 27: 812–6
Reiner NE, Bloxham DD, Thompson WL, et al. Nephrotoxicity of gentamicin and tobramycin given single daily or continu-ously in dogs. J Antimicrob Chemother 1978; 4 Suppl. A: 85–101
Luft FC, Patel V, Yum MN, et al. Experimental aminoglycoside nephrotoxicity. J Lab Clin Med 1975; 86: 213–20
Frame PT, Phair JP, Watanakunakorn C, et al. Pharmacologic factors associated with gentamicin nephrotoxicity in rabbits. J Infect Dis 1977; 135: 952–6
Bennett WA, Plamp CE, Gilbert DN, et al. The influence of dosage regimen on experimental gentamicin nephrotoxicity: dissociation of peak serum concentrations from renal failure. J Infect Dis 1979; 140: 576–80
Olier B, Viotte G, Morin JP, et al. Influence of dosage regimen on experimental tobramycin nephrotoxicity. Chemotherapy 1983; 29: 385–94
Davis RR, Brummett RE, Bendrick TW, et al. Dissociation of maximum concentration of kanamycin in plasma and perilymph from ototoxic effect. J Antimicrob Chemother 1984; 14: 291–302
Bamonte F, Dionisotti S, Gambia M, et al. Relation of dosing regimen to aminoglycoside ototoxicity: evaluation of auditory damage in the guinea pig. Chemotherapy 1990; 36: 41–50
Takumida M, Nishida I, Nikaido M, et al. Effect of dosing schedule on aminoglycoside ototoxicity: comparative cochlear ototoxicity of amikacin and isepamicin. J Otorhinolaryngology 1990; 52: 341–9
Kahlmeter G, Dahlager JI. Aminoglycoside ototoxicity — a review of clinical studies published between 1975 and 1982. J Antimicrob Chemother 1984; 13 Suppl. A: 9–22
Ohtani I, Ohtsuki K, Aikawa T, et al. Ototoxicity of aminogly-coside antibiotics by rapid intravenous injection. Nippon Jibiinkoka Gakkai Kaiho 1980; 83: 1482–90
Brummett RE, Fox KE, Bendrick TW, et al. Ototoxicity of tobramycin, gentamicin, amikacin and sisomicin in the guinea pig. J Antimicrob Chemother 1978; 4 Suppl. A: 73–84
Aran JM, Erre JP, Guilhaume A, et al. The comparative ototoxicities of gentamicin, tobramycin and dibekacin in the guinea pig. A functional and morphological cochlear and vestibular study. Acta Otolaryngol 1982; 390: 1–30
Tran Ba Huy, Deffrennes D. Aminoglycoside ototoxicity: influence of dosage regimen on drug uptake and correlation between membrane binding and some clinical features. Acta Otolaryngol 1988; 105: 511–5
Federspil P, Schatzle W, Tiesler E. Pharmacokinetics and ototoxicity of gentamicin, tobramycin, and amikacin. J Infect Dis 1976; 134 Suppl.: S200–5
Tran Ba Huy, Manuel C, Meulemans A, et al. Pharmacokinetics of gentamicin in perilymph and endolymph of the rat as determined by radioimmunoassay. J Infect Dis 1981; 143: 476–86
Maller R, Emanuelsson BM, Isaksson B, et al. Amikacin once daily: a new dosing regimen based on drug pharmacokinetics. Scand J Infect Dis 1990; 22: 575–9
Konrad F, Wagner R, Neumeister B, et al. Studies on drug monitoring in thrice and once daily treatment with aminoglycosides. Intensive Care Med 1993; 19: 215–20
Valcke YJ, Vogelaers DP, Colardyn FA, et al. Penetration of netilmicin in the lower respiratory tract after once daily dosing. Chest 1992; 101: 1028–32
Skopnik H, Wallraf R, Nies B, et al. Pharmacokinetics and an-tibacterial activity of daily gentamicin. Arch Dis Child 1992; 67: 57–61
Pierre C, Blanchet F, Seta N, et al. Tolerance of once-daily dosing of netilmicin and teicoplanin, alone or in combination, in healthy volunteers. Clin Pharmacol Ther 1988; 44: 458–66
Marik PE, Havlik I, Monteagudo FSE, et al. The pharmacoki-netics of amikacin in critically ill adult and paediatric patients: comparison of once- versus twice-daily dosing regimens. J Antimicrob Chemother 1991; 27 Suppl. C: 81–9
Zaske DE. Gentamicin pharmacokinetics in 1,640 patients: method for control of serum concentrations. Antimicrob Agents Chemother 1982; 2: 407–11
Principi N, Gervasoni A, Reali E, et al. Treatment of urinary tract infections in children with a single daily dose of gentamicin. Helv Paediatr Acta 1977; 32: 343–50
Beaucaire G, Leroy O, Beuscart C, et al. Clinical and bacteriological efficacy and the practical aspects of amikacin given once daily for severe infections. J Antimicrob Chemother 1991; 27 Suppl. C: 91–103
Kafetzis DA, Sianidou L, Vlachos E, et al. Clinical and pharmacokinetic study of a single daily dose of amikacin in paediatric patients with severe gram-negative infections. J Antimicrob Chemother 1991; 27 Suppl. C: 105–12
Trujillo H, Robledo J, Robledo C, et al. Single daily dose amikacin in paediatric patients with severe gram negative infections. J Antimicrob Chemother 1991; 27 Suppl. C: 141–7
Martino P, Girmenia C, Raccah R, et al. Ceftriaxone and amikacin as single daily dose in the empiric therapy for febrile episodes in neutropenic patients. Haematologica 1990; 75: 69–74
Meunier F, Van der Auwera P, Aoun M, et al. Empirical antimicrobial therapy with a single daily dose of ceftriaxone plus amikacin in febrile granulocytopenic patients: a pilot study. J Antimicrob Chemother 1991; 27 Suppl. C: 129–39
Viscoli C, Dudley M, Ferrea G, et al. Serum levels and safety of single daily dosing of amikacin in children undergoing bone marrow transplantation. J Antimicrob Chemother 1991; 27 Suppl. C: 113–9
Martino P, Girmenia C, Raccah R, et al. Single daily dose ceftriaxone plus amikacin treatment of febrile episodes in neutropenic patients attending day hospital for hematologic malignancies. Oncology 1992; 49: 49–52
PC-Size: consultant program for sample size determinations. The American Statistician 1990; 44: 243
Borzak S, Ridker PM. Discordance between meta-analyses and large-scale randomized controlled trials. Ann Intern Med 1995; 123: 873–7
Reesor Nimmo C, Mamdani F, Baker A, et al. Development and implementation of simplified aminoglycoside empiric dosing guidelines. Pharmacotherapy 1993; 13: 408–14
Gibaldi M, Perrier D, editors. Pharmacokinetics. 2nd ed. New York: Marcel Dekker Inc. 1982: 385–403
Martinusen S, Chen D, Frighetto L, et al. Comparison of cefoxitin and ceftizoxime in a hospital therapeutic interchange program. Can Med Assoc J 1993; 148(7): 1161–9
Frighetto L, Nickoloff D, Jewesson P. Antibiotic therapeutic interchange program: six years of experience. Hosp Formul 1995; 30(2): 92–105
Shalansky S, Gupta S, Jewesson P. Impact of a practical two-stage intervention on aminoglycoside usage. Hosp Formul 1989; 24: 332–41
Jewesson PJ. Economic impact of intravenous-to-oral antibacterial stepdown therapy. Clin Drug Invest 1996; 11: S1–9
Hitt CM, Klepser ME, Nightingale CH, et al. Pharmacoeconomic impact of a once-daily aminoglycoside program [abstract no. 107]. 35th Interscience Conference on Antimicrobial Agents and Chemotherapy: 1995 Sep 17–20; San Francisco (CA)
Rotschafer JC, Rybak MJ. Single daily dosing of aminoglycosides: a commentary. Ann Pharmacother 1994; 28: 797–801
Gin AS, Ariano RE. Comment: single daily dosing of aminoglycosides. Ann Pharmacother 1994; 28: 1412
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marra, F., Partovi, N. & Jewesson, P. Aminoglycoside Administration as a Single Daily Dose. Drugs 52, 344–370 (1996). https://doi.org/10.2165/00003495-199652030-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-199652030-00003