Summary
Dexketoprofen trometamol is a water-soluble salt of the dextrorotatory enantiomer of the nonsteroidal anti-inflammatory drug (NSAID) ketoprofen. Racemic ketoprofen is used as an analgesic and an anti-inflammatory agent, and is one of the most potent in vitro inhibitors of prostaglandin synthesis. This effect is due to the S(+)-enantiomer (dexketoprofen), while the R(−)-enantiomer is devoid of such activity.
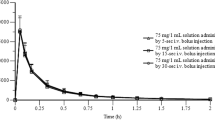
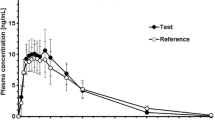
The pharmacokinetic profile of ketoprofen and its enantiomers was assessed in several animal species and in human volunteers. In humans, the relative bioavailability of oral dexketoprofen trometamol (12.5 and 25mg, respectively) is similar to that of oral racemic ketoprofen (25 and 50mg, respectively), as measured in all cases by the area under the concentration-time curve values for S(+)-ketoprofen. Dexketoprofen trometamol, given as a tablet, is rapidly absorbed, with a time to maximum plasma concentration (tmax) of between 0.25 and 0.75 hours, whereas the tmax for the S-enantiomer after the racemic drug, administered as tablets or capsules prepared with the free acid, is between 0.5 and 3 hours. Peak plasma concentrations of 1.4 and 3.1 mg/L are reached after administration of dexketoprofen trometamol 12.5 and 25mg, respectively.
From 70 to 80% of the administered dose is recovered in the urine during the first 12 hours, mainly as the acyl-glucuronoconjugated parent drug. No R(−)-ketoprofen is found in the urine after administration of dexketoprofen [S(+)-ketoprofen], confirming the absence of bioinversion of the S(+)-enantiomer in humans.
In animal studies, the anti-inflammatory potency of dexketoprofen was always equivalent to that demonstrated by twice the dose of ketoprofen. Similarly, animal studies showed a high analgesic potency for dexketoprofen trometamol. The R(−)-enantiomer demonstrated a much lower potency, its analgesic action being apparent only in conditions where the metabolic bioinversion to the S(+)-enantiomer was significant.
The gastric ulcerogenic effects of dexketoprofen at various oral doses (1.5 to 6 mg/kg) in the rat do not differ from those of the corresponding double doses (3 to 12 mg/kg) of racemic ketoprofen. Repeated (5-day) oral administration of dexketoprofen as the trometamol salt causes less gastric ulceration than was observed after the acid form of both dexketoprofen and the racemate. In addition, single dose dexketoprofen as the free acid at 10 or 20 mg/kg does not show a significant intestinal ulcerogenic effect in rats, while racemic ketoprofen 20 or 40 mg/kg is clearly ulcerogenic to the small intestine.
The analgesic efficacy of oral dexketoprofen trometamol 10 and 20mg is superior to that of placebo and similar to that of ibuprofen 400mg in patients with moderate to severe pain after third molar extraction. The time to onset of pain relief appeared to be shorter in patients treated with dexketoprofen trometamol than in those treated with ibuprofen 400mg. Dexketoprofen trometamol was well tolerated, with a reported incidence of adverse events similar to that of placebo.
Similar content being viewed by others
References
Veys EM. 20 years’ experience with ketoprofen. Scand J Rheumatol Suppl. 1991; 90: 1–44
Caldwell J, Hutt AJ, Fournel-Gigleux S. The metabolic chiral inversion and dispositional enantioselectivity of the 2-arylpropionic acids and their biological consequences. Biochem Pharmacol 1988; 37: 105–14
Cooper SA. Ketoprofen in oral surgery pain: a review. J Clin Pharmacol 1988; 28: S40–6
Hayball PJ, Nation RL, Bochner F. Enantioselective pharmaco-dynamics of the nonsteroidal anti-inflammatory drug ketoprofen: in vitro inhibition of human platelet cyclo-oxygenase activity. Chirality 1992; 4: 484–7
Avouac B, Teule M. Ketoprofen: the European experience. J Clin Pharmacol 1988; 28: S2–7
McCormack K, Urquhart E. Correlation between nonsteroidal anti-inflammatory drug efficacy in a clinical pain model and the dissociation of their anti-inflammatory and analgesic properties in animal models. Clin Drug Invest 1995; 9: 88–97
Lotsch J, Geisslinger G, Mohammadian P, et al. Effects of flurbiprofen enantiomers on pain-related chemosomatosens-ory evoked potentials in human subjects. Br J Clin Pharmacol 1995; 40: 339–46
Neugebauer V, Geisslinger G, Rumenapp P, et al. Antinociceptive effects of R(−) and S(+)-flurbiprofen on rat spinal dorsal horn neurons rendered hyperexcitable by an acute knee joint inflammation. J Pharmacol Exp Ther 1995; 275: 618–28
Williams RL, Upton RA. The clinical pharmacology of ketoprofen. J Clin Pharmacol 1988; 28: S13–22
Jamali F, Brocks DR. Clinical pharmacokinetics of ketoprofen and its enantiomers. Clin Pharmacokinet 1990; 19: 197–217
Abas A, Meffin PJ. Enantioselective disposition of racemic ketoprofen in rabbits with normal and diminished renal function. Clin Exp Pharmacol Physiol 1985; Suppl. 9: 41–2
Abas A, Meffin PJ. Enantioselective disposition of 2-arylpropionic acid nonsteroidal anti-inflammatory drugs. IV. Ketoprofen disposition. J Pharmacol Exp Ther 1987; 240: 637–41
Foster RT, Jamali F. Stereoselective pharmacokinetics of ketoprofen in the rat. Influence of route of administration. Drug Metab Dispos 1988; 16: 623–6
Foster RT, Jamali F, Russell AS, et al. Pharmacokinetics of ketoprofen enantiomers in healthy subjects following single and multiple doses. J Pharm Sci 1988; 77: 70–3
Foster RT, Jamali F, Russell AS, et al. Pharmacokinetics of ketoprofen enantiomers in young and elderly arthritic patients following single and multiple doses. J Pharm Sci 1988; 77: 191–5
Foster RT, Jamali F, Russell AS. Ketoprofen enantiomers in synovial fluid. J Pharm Sci 1989; 78: 881–2
Foster RT, Jamali F, Russell AS. Pharmacokinetics of ketoprofen enantiomers in cholecystectomy patients: influence of probenecid. Eur J Clin Pharmacol 1989; 37: 589–94
Stiegler S, Birkel M, Jost V, et al. Pharmacokinetics and relative bioavailability after single dose administration of 25 mg ketoprofen solution as compared to tablets. Methods Find Exp Clin Pharmacol 1995; 17: 129–34
Geisslinger G, Menzel S, Wissel K, et al. Pharmacokinetics of ketoprofen enantiomers after different doses of the racemate. Br J Clin Pharmacol 1995; 40: 73–5
Iwakawa S, He X, Hashimoto S, et al. Stereoselective disposition of ketoprofen in rats. Drug Metab Dispos 1991; 19: 717–8
Menzel S, Beck WS, Brune K, et al. Stereoselectivity of biliary excretion of 2-arylpropionates in rats. Chirality 1993; 5: 422–7
Menzel S, Sauernheimer C, Brune K, et al. Is the inversion from R- to S-ketoprofen concentration dependent? Investigations in rats in vivo and in vitro. Biochem Pharmacol 1994; 47: 1267–70
Jamali F, Russell AS, Foster RT, et al. Ketoprofen pharmacokinetics in humans: evidence of enantiomeric inversion and lack of interaction. J Pharm Sci 1990; 79: 460–1
Jamali F, Mehvar R, Pasutto FM. Enantioselective aspects of drug action and disposition: therapeutic pitfalls. J Pharm Sci 1989; 78: 695–715
Mauleón D, Mis R, Ginesta J, et al. Pharmacokinetics of ketoprofen enantiomers in monkeys following single and multiple oral administration. Chirality 1994; 6: 537–42
Mis R, Tost D, Ortega E, et al. Pharmacokinetics in plasma of oral 14C-LM-1158. TRIS and 14C-R-(−)-ketoprofen in several species. 1993. Report codes: PK6/LM-1158. TRIS/93; PK7/LM-1158. TRIS/93; PK11/LM-1158. TRIS/93.
Cochet P, Bromet-Petit M, Mis R, et al. Quantitative tissue distribution of LM-1158 in Long-Evans rats after a single (5 mg/kg) oral administration. 5th European International Society for the Study of Xenobiotics Meeting. 1993 Sep 26-29: Tours, France
Muller N, Payan E, Lapicque F, et al. Pharmacological aspects of chiral nonsteroidal anti-inflammatory drugs. Fundam Clin Pharmacol 1990; 4: 617–34
Hayball PJ, Nation RL, Bochner F, et al. Plasma protein binding of ketoprofen enantiomers in man: method development and its application. Chirality 1991; 3: 460–6
Dubois N, Lapicque F, Abiteboul M, et al. Stereoselective protein binding of ketoprofen: effect of albumin concentration and of the biological system. Chirality 1993; 5: 126–134
Dubois N, Muller N, Lapicque F, et al. Stereoselective protein binding of nonsteroidal anti-inflammatory drugs: pharmacological consequences. Therapie 1993; 48: 335–9
Hayball PJ, Nation RL, Bochner F, et al. The influence of renal function on the enantioselective pharmacokinetics and phar-macodynamics of ketoprofen in patients with rheumatoid arthritis. Br J Clin Pharmacol 1993; 36: 185–93
Hayball PJ, Nation RL, Bochner F. Stereoselective interactions of ketoprofen glucuronides with human plasma protein and serum albumin. Biochem Pharmacol 1992; 44: 291–9
Dubois N, Lapicque F, Maurice MH, et al. In vitro irreversible binding of ketoprofen glucuronide to plasma proteins. Drug Metab Dispos 1993; 21: 617–23
Spahn-Langguth H, Benet LZ. Acyl glucuronides revisited: is the glucuronide process a toxification as well as a detoxification mechanism? Drug Metab Rev 1992; 24: 5–47
Dubois-Presle N, Lapicque F, Maurice MH, et al. Stereoselective esterase activity of human serum albumin toward ketoprofen glucuronide. Mol Pharmacol 1995; 47: 647–53
Mis R, Ginesta J, Vidal M, et al. Pharmacokinetics of enantiomers R-(−)-ketoprofen and LM-1158 after single oral administration of racemic 14C-ketoprofen. TRIS to mice. 1993. Report code: PK13/LM-1158. TRIS/93
Yasui H, Yamaoka K, Dote N, et al. Moment analysis of stereoselective biliary excretion and chiral inversion of ketoprofen enantiomers in perfused rat liver. J Pharm Sci 1995; 84: 1327–31
Populaire P, Terlain B, Pascal S, et al. Biological behaviour: plasmatic levels, excretion and biotransformation of 2-(3-benzoylphenyl)propionic acid (ketoprofen) in animals and man. Ann Pharm Fr 1973; 31: 735–49
Delbarre F, Roucayrol JC, Amor B, et al. Pharmacokinetic study of ketoprofen (19.583 RP) in man using the tritiated compound. Scand J Rheumatol Suppl. 1976; 14: 45–52
Artigas R, Barbanoj MJ, Gich I. Pharmacokinetic study of LM-1158. Comparison of the relative bioavailability of two different formulations of LM-1158 and ketoprofen after a single oral dose administration in healthy volunteers. 1993. Report code: FC8/LM-11588/92
Jamali F. Pharmacokinetics of enantiomers of chiral nonsteroidal anti-inflammatory drugs. Eur J Drug Metab Pharmacokinet 1988; 13: 1–9
Mayer JM. Stereoselective metabolism of anti-inflammatory 2-arylpropionates. Acta Pharm Nord 1990; 2: 197–216
Wechter WJ. Drug chirality: on the mechanism of R-aryl propionic acid class NSAIDs. Epimerization in humans and the clinical implications for the use of racemates. J Clin Pharmacol 1994; 34: 1036–42
Nakamura Y, Yamaguchi T, Takahashi S, et al. Optical isomerization mechanism of R-(−)-hydratropic acid derivatives. J Pharmacobio Dyn 1980; 4: S–1
Caldwell J. Xenobiotic acyl-coenzymes A: critical intermediates in the biochemical pharmacology and toxicology of car-boxylic acids. Biochem Soc Trans 1984; 12: 9–11
Reichel C, Bang H, Brune K, et al. 2-arylpropionyl-CoA epimerase: partial peptide sequences and tissue localization. Biochem Pharmacol 1995; 50: 1803–6
Benoit E, Delatour P, Olivier L, et al. (−)-R-fenoprofen: formation of fenoprofenyl-coenzyme A by rat liver microsomes. Biochem Pharmacol 1995; 49: 1717–20
Soraci A, Benoit E. In vitro fenoprofenyl-coenzyme A thioester formation: interspecies variations. Chirality 1995; 7: 534–40
Tanaka Y, Shimomura Y, Hirota T, et al. Formation of glycine conjugate and (−)-(R)-enantiomer from (+)-(S)-2-phenylpropionic acid suggesting the formation of the CoA thioester intermediate of (+)-(S)-enantiomer in dogs. Chirality 1992; 4: 342–8
King J, Mauron C, LeGoff C, et al. Bidirectional chiral inversion of the enantiomers of the nonsteroidal anti-inflammatory drug oxindanac in dogs. Chirality 1994; 6: 460–6
Mis R, Tost D, Ortega E, et al. Bioinversion of ketoprofen enantiomers in several species. Methods Find Exp Clin Pharmacol 1994; 16 Suppl. 1: 81
Carabaza A, Suesa N, Tost D, et al. Stereoselective metabolic pathways of ketoprofen in the rat: incorporation into triacylglycerols and enantiomeric inversion. Chirality 1996; 8: 163–72
Menzel-Soglowek S, Geisslinger G, Mollenhauer J, et al. Metabolic chiral inversion of 2-arylpropionates in rat H4IIE and human Hep G2 hepatoma cells. Relationship to in vivo metabolism. Biochem Pharmacol 1992; 43: 1487–92
Aberg G, Ciofalo VB, Pendleton R, et al. Inversion of (R)-to (S)-ketoprofen in eight animal species. Chirality 1995; 7: 383–7
Gich I, Bayes M, Barbanoj MJ, et al. Bioinversion of R (−)-ketoprofen following oral administration in healthy volunteers. Clin Drug Invest 1996; 11: 347–53
Williams KM. Enantiomers in arthritic disorders. Pharmacol Ther 1990; 46: 273–95
Williams K, Day R, Knihinicki R, et al. The stereoselective uptake of ibuprofen enantiomers into adipose tissue. Biochem Pharmacol 1986; 35: 3403–5
Sallustio BC, Meffin PJ, Knights KM. The stereospecific incorporation of fenoprofen into rat hepatocyte and adipocyte triacylglycerols. Biochem Pharmacol 1988; 37: 1919–23
Zhao B, Geisslinger G, Hall I, et al. The effect of the enantiomers of ibuprofen and flurbiprofen on the beta-oxidation of palmitate in the rat. Chirality 1992; 4: 137–41
Knights KM, Drew R. The effects of ibuprofen enantiomers on hepatocyte intermediary metabolism and mitochondrial respiration. Biochem Pharmacol 1992; 44: 1291–6
Roberts BJ, Knights KM. Inhibition of rat peroxisomal palmitoyl-CoA ligase by xenobiotic carboxylic acids. Biochem Pharmacol 1992; 44: 261–7
Mayer JM, Roydevis M, Audergon C, et al. Interactions of antiinflammatory 2-arylpropionates (profens) with the metabolism of fatty acids: in vitro studies. Int J Tissue React 1994; 16: 59–72
Sallustio BC, Knights KM, Meffin PJ. The stereospecific inhibition of endogenous triacylglycerol synthesis by fenoprofen in rat isolated adipocytes and hepatocytes. Biochem Pharmacol 1990; 40: 1414–7
Gich I, Barbanoj MJ, Artigas R, et al. New fast-onset oral formulation of dexketoprofen. 6th INWIN′95 (Interscience World Conference on Inflammation, Antirheumatics, Analgesics and Immunomodulators). 1995 Mar 28-30: Geneva
Evans AM. Enantioselective pharmacodynamics and pharma-cokinetics of chiral non-steroidal anti-inflammatory drugs. Eur J Clin Pharmacol 1992; 42: 237–56
Xie W, Robertson DL, Simmons DL. Mitogen-inducible pros-taglandin G/H synthase: a new target for nonsteroidal anti-inflammatory drugs. Drug Rev Res 1992; 25: 249
Jones DA, Carlton DP, McIntyre TM, et al. Molecular cloning of human prostaglandin endoperoxide synthase type II and demonstration of expression in response to cytokines. J Biol Chem 1993; 268: 9049–54
Seibert K, Zhang Y, Leahy K, et al. Pharmacological and biochemical demonstration of the role of cyclooxygenase 2 in inflammation and pain. Proc Natl Acad Sci USA 1994; 91: 12013–7
Vane JR, Mitchell JA, Appleton I, et al. Inducible isoforms of cyclo-oxygenase and nitric oxide synthase in inflammation. Proc Natl Acad Sci USA 1994; 91: 2046–50
Vane JR, Botting RM. New insights into the mode of action of anti-inflammatory drugs. Inflamm Res 1995; 44: 1–10
Dray A. Inflammatory mediators of pain. Br J Anaesth 1995; 75: 125–31
Rang HP, Urban L. New molecules in analgesia. Br J Anaesth 1995; 75: 145–56
Mitchell JA, Akarasereenont P, Thiemermann C, et al. Selectivity of nonsteroidal anti-inflammatory drugs as inhibitors of constitutive and inducible cyclooxygenase. Proc Natl Acad Sci USA 1993; 90: 11693–7
Meade EA, Smith WL, DeWitt DL. Differential inhibition of prostaglandin endoperoxide synthase (cyclooxygenase) isozymes by aspirin and other non-steroidal anti-inflammatory drugs. J Biol Chem 1993; 268: 6610–4
Tavares IA, Bishai PM, Bennett A. Activity of nimesulide on constitutive and inducible cyclooxygenases. Arzneimittel Forschung 1995; 45: 1093–5
Glaser K, Sung ML, O’Neill K, et al. Etodolac selectively inhibits human prostaglandin G/H synthase 2 (PGHS-2) versus human PGHS-1. Eur J Pharmacol 1995; 281: 107–11
Cipollone F, Ganci A, Panara MR, et al. Effects of nabumetone on prostanoid biosynthesis in humans. Clin Pharmacol Ther 1995; 58: 335–41
Vane JR, Botting RM. The mode of action of anti-inflammatory drugs. Postgrad Med 1990; 66 Suppl. 4: S2–17
Gaut ZN, Baruth H, Randall LO, et al. Stereoisomeric relationship among anti-inflammatory activity, inhibition of platelet aggregation, and inhibition of prostaglandin synthetase. Prostaglandins 1975; 10: 59–66
Ku EC, Wasvary JM. Inhibition of prostaglandin synthase by pirprofen. Studies with sheep seminal vesicle enzyme. Biochim Biophys Acta 1975; 384: 360–8
Adams SS, Bresloff P, Mason CG. Pharmacological differences between the optical isomers of ibuprofen: evidence for metabolic inversion of (−)-R isomer. J Pharm Pharmacol 1976; 28: 256–7
Buttinoni A, Ferrari M, Colombo M, et al. Biological activity of indoprofen and its optical isomers. J Pharm Pharmacol 1983; 35: 603–4
Guzman A, Yuste F, Toscano RA, et al. Absolute configuration of (−)-5-benzoyl-1,2-dihydro-3H-pyrrolo[ 1,2α]pyrrole-1-carboxylic acid, the active enantiomer of ketorolac. J Med Chem 1986; 29: 589–91
Yamaguchi T, Hirose K, Nakamura Y, et al. The inhibitory activities of 480156-S and its related compounds on prostaglandin synthetase. Folia Pharmacol Jpn 1987; 90: 295–302
Patrignani P, Volpi D, Ferrario R, et al. Effects of racemic, S-and R-indobufen on cyclooxygenase and lipoxygenase activities in human whole blood. Eur J Pharmacol 1990; 191: 83–8
Cerletti C, Manarini S, Colombo M, et al. The (+)enantiomer is responsible for the antiplatelet and anti-inflammatory activity of (±)-indobufen. J Pharm Pharmacol 1990; 42: 885–7
Evans AM, Nation RL, Sansom LN, et al. Effect of racemic ibuprofen dose on the magnitude and duration of platelet cyclooxygenase inhibition: relationship between inhibition of thromboxane production and the plasma unbound concentration of S(+)-ibuprofen. Br J Clin Pharmacol 1991; 31: 131–8
Moreno JJ, Calvo L, Fernandez F, et al. Biological activity of ketoprofen and its optical isomers. Eur J Pharmacol 1990; 183: 2263–4
Brune K, Beck WS, Geisslinger G, et al. Aspirin-like drugs may block pain independently of prostaglandin synthesis inhibition. Experientia 1991; 47: 257–61
Suesa N, Fernandez MF, Gutierrez M, et al. Stereoselective cyclooxygenase inhibition in cellular models by the enantiomers of ketoprofen. Chirality 1993; 5: 589–95
Moreno JJ, Calvo L, Fernandez F, et al. Biological activity of ketoprofen and its optical isomers. XIth International Congress of Pharmacology. 1990 Jul 1: Amsterdam
Villanueva M, Heckenberger R, Strobach H, et al. Equipotent inhibition by R(−)-, S(+)- and racemic ibuprofen of human polymorphonuclear cell function in vitro. Br J Clin Pharmacol 1993; 35: 235–42
Panara MR, Greco A, Santini G, et al. Effects of the novel anti-inflammatory compounds, N-[2-(cyclohexyloxy)-4-nitrophenyl]methanesulphonamide (NS-398) and 5-meth-ane- sulphonamido-6-(2,4-difluorothiophenyl)-1 -indanone (L745,337), on the cyclo-oxygenase activity of human blood prostaglandin endoperoxide synthases. Br J Pharmacol 1995; 116: 2429–34
Carabaza A, Cabré F, Rotllán E, et al. Stereoselective inhibition of inducible cyclooxygenase by chiral non-steroidal anti-in-flammatory drugs. J Clin Pharmacol. In press
Ferrer X, Fernández MF, Calvo L, et al. Anti-inflammatory activity of S-(+)-ketoprofen in the carrageenan-induced paw edema of the rat. Methods Find Exp Clin Pharmacol 1994; 16 Suppl. 1: 82
Woolf CJ. A new strategy for the treatment of inflammatory pain. Prevention or elimination of central sensitisation. Drugs 1994; 47: 1–9
Brune K. Spinal cord effects of antipyretic analgesics. Drugs 1994; 47: 21–7
McCormack K. The spinal actions of nonsteroidal anti-inflammatory drugs and the dissociation between their anti-inflammatory and analgesic effects. Drugs 1994; 47: 28–45
McCormack K. Non-steroidal anti-inflammatory drugs and spinal nociceptive processing. Pain 1994; 59: 9–43
Cashman J, McAnulty G. Nonsteroidal anti-inflammatory drugs in perisurgical pain management. Mechanisms of action and rationale for optimum use. Drugs 1995; 49: 51–70
Jurna I. Acetylsalicylic acid and related compounds depress nociceptive activity in the thalamus by a central action: indications for the involvement of prostaglandins. Prog Pharmacol Clin Pharmacol 1993; 10: 51–68
Yaksh TL, Malmberg AB. Antinociceptive actions of spinal nonsteroidal anti-inflammatory agents on the formalin test in the rat. J Pharmacol Exp Ther 1992; 263: 136–46
Yaksh TL, Malmberg AB. Spinal actions of NSAIDs in blocking spinally mediated hyperalgesia: the role of cyclooxygenase products. Agents Actions Suppl. 1993; 41: 89–100
Brune K, Menzel-Soglowek S, Zeilhofer HU. Differential analgesic effects of aspirin-like drugs. Drugs 1992; 44: 52–9
Urquhart E. Central analgesic activity of nonsteroidal anti-inflammatory drugs in animal and human pain models. Semin Arthritis Rheum 1993; 23: 198–205
Rice ASC, Lloyd J, Bullingham RES, et al. Ketorolac penetration into the cerebrospinal fluid of humans. J Clin Anesth 1993; 5: 459–62
Bannwarth B, Lapicque F, Pehourcq F, et al. Stereoselective disposition of ibuprofen enantiomers in human cerebrospinal fluid. Br J Clin Pharmacol 1995; 40: 266–9
Netter P, Lapicque F, Bannwarth B, et al. Diffusion of intramuscular ketoprofen into the cerebrospinal fluid. Eur J Clin Pharmacol 1985; 29: 319–21
Malmberg A, Yaksh TL. Cyclooxygenase inhibition and the spinal release of prostaglandin E2 and amino acids evoked by paw formalin injection: a microdialysis study in unanesthe-tized rats. J Neurosci 1995; 15: 2768–76
Malmberg A, Yaksh TL. Antinociception produced by spinal delivery of the S and R enantiomers of flurbiprofen in the formalin test. Eur J Pharmacol 1994; 256: 205–9
Knihinicki RD, Day RO, Graham GG, et al. Stereoselective disposition of ibuprofen and flurbiprofen in rats. Chirality 1990; 2: 134–40
Peskar BM, Kluge S, Peskar BA, et al. Effects of pure enantiomers of flurbiprofen in comparison to racemic flurbiprofen on eicosanoid release from various rat organs ex vivo. Prostaglandins 1991; 42: 515–31
Sunshine A, Zighelboim J, Olson N, et al. Flurbiprofen, flurbiprofen dextrorotatary component (BTS 24332), and placebo in post episiotomy pain. Clin Pharmacol Ther 1987; 42: 162
Fernandez MF, Ferrer X, Calvo L, et al. Analgesic activity of S-(+)-ketoprofen in the abdominal pain induced by phenylbenzoquinone in the mouse. Methods Find Exp Clin Pharmacol 1994; 16 Suppl. 1: 83
Jaques R. Antagonism of non-steroidal anti-inflammatory drugs and narcotic analgesics against ethacrynic acid induced writhing. Arzneimittel Forschung 1977; 27: 1698–700
Calvo L, Fernandez MF, Ferrer X, et al. Analgesic activity of ketoprofen enantiomers in the abdominal pain induced by in-traperitoneal injection of etacrinic acid in the rat. Methods Find Exp Clin Pharmacol 1994; 16 Suppl. 1: 83
Speirs CJ. Comparison of the safety of several nonsteroidal anti-inflammatory drugs currently or formerly marketed in the United Kingdom. J Clin Pharmacol 1988; 28: S8–12
Meryn S. Nonsteroidal anti-inflammatory drugs and peptic ulcers — mechanisms, risk factors and treatment. Clin Exp Rheumatol 1994; 12: 119–21
Committee on Safety of Medicines update. Non steroidal anti-inflammatory drugs and serious gastrointestinal adverse reactions. BMJ 1986; 292: 614–9
Wright V, Elshal WS, Hopkins R, et al. NSAID gastropathy in rheumatology: an audit and review of the literature. J Drug Develop Clin Practice 1995; 7: 21–9
Armstrong CP, Blower AL. Non-steroidal anti-inflammatory drugs and life threatening complications of peptic ulceration. Gut 1987; 28: 527–32
Bateman DN. NSAIDs: time to re-evaluate gut toxicity. Lancet 1994; 343: 1051–2
Rainsford KD. Mechanisms of gastrointestinal toxicity of nonsteroidal anti-inflammatory drugs. Scand J Gastroenterol 1989; 24 Suppl. 163: 9–16
Ligumsky M, Sestieri M, Zimmerman J, et al. Rectal administration of nonsteroidal anti-inflammatory drugs. Effect on rat gastric ulcerogenicity and prostaglandin E2 synthesis. Gastroenterology 1990; 98: 1245–9
Beck WS, Schneider HT, Dietzel K, et al. Gastrointestinal ulceration induced by anti-inflammatory drugs in rats. Arch Toxicol 1990; 64: 210–7
Brune K. Is there a rational basis for the different spectra of adverse effects of nonsteroidal anti-inflammatory drugs (NSAIDs)?. Drugs 1990; 40 Suppl. 5: 12–5
Wallace JL, Keenan CM, Granger N. Gastric ulceration induced by nonsteroidal anti-inflammatory drugs is a neutrophil dependent process. Am J Physiol 1990; 359: G462–7
Taha AS, Sturrock RD, Russell RI. Mucosal erosions in longterm non-steroidal anti-inflammatory drug users: predisposition to ulceration and relation to Helicobacter pylori. Gut 1995; 36: 334–6
Mizokami Y, Tamura K, Fukuda Y, et al. Non-steroidal anti-inflammatory drugs associated with gastroduodenal injury and Helicobacter pylori. Eur J Gastroenterol Hepatol 1994; 6: S109–12
Rainsford K. The comparative gastric ulcerogenic activities of non-steroid anti-inflammatory drugs. Agents Actions 1977; 7: 573–7
Dearden JC, Nicholson RM. Correlation between gastric irritancy and anti-inflammatory activity of non-steroidal anti-inflammatory drugs. J Pharm Pharmacol 1984; 36: 713–5
Bergmann JF, Chassany O, Geneve J, et al. Endoscopic evaluation of the effect of ketoprofen, ibuprofen and aspirin on the gastroduodenal mucosa. Eur J Clin Pharmacol 1992; 42: 685–8
Savage RL, Moller PW, Ballantyne CL, et al. Variation in the risk of peptic ulcer complications with nonsteroidal anti-inflammatory drug therapy. Arthritis Rheum 1993; 36: 84–90
Lanza LL, Walker AM, Bortnichak EA, et al. Peptic ulcer and gastrointestinal haemorrhage associated with nonsteroidal anti-inflammatory drug use in patients younger than 65 years: a large health maintenance organization cohort study. Arch Intern Med 1995; 155: 1371–7
Langman MJ, Weil J, Lawson DH, et al. Risks of bleeding peptic ulcer associated with individual non-steroidal anti-inflammatory drugs. Lancet 1994; 343: 1075–8
Garcia Rodriguez LA, Jick H. Risk of upper gastrointestinal bleeding and perforation associated with individual nonsteroidal anti-inflammatory drugs. Lancet 1994; 343: 769–72
Wechter WJ, Bigornia A, Murray ED, et al. Rac-flurbiprofen is more ulcerogenic than its S-enantiomer. Chirality 1993; 5: 492–4
Fernández MF, Calvo L, Ferrer X, et al. Study of gastric damage caused by single oral administration of LM-1158. TRIS and LM-1158 suspension in rats. 1993. Report codes: PHAR5/-LM-1158/92;PHAR17/LM-1158.TRIS/93
Fernández MF, Calvo L, Ferrer X, et al. Study of gastric damage caused by repeated oral administration of LM-1158.TRIS and LM-1158 suspension in rats. 1993. Report code: PHAR6/LM-1158/92; PHAR18/LM-1158.TRIS/93
Sjarnason I, MacPherson AJS. Intestinal toxicity of nonsteroidal anti-inflammatory drugs. Pharmacol Ther 1994; 62: 145–7
Levi S, Shaw-Smith C. Non-steroidal anti-inflammatory drugs: how do they damage the gut? Br J Rheumatol 1994; 33: 605–12
Hochain P, Colin R. Side effects of non-steroidal anti-inflammatory drugs on the small and large intestine. Gastroenterol Clin Biol 1995; 19: B79–83
Bidlingmaier A, Hammermaier A, Nagyivanyi P, et al. Gastrointestinal blood loss induced by three different nonsteroidal anti-inflammatory drugs. Arzneimittel Forschung 1995; 45: 491–3
Myllykangas-Luosujarvi R, Aho K, Isomaki H. Death attributed to antirheumatic medication in a nationwide series of 1666 patients with rheumatoid arthritis who have died. J Rheumatol 1995; 22: 2214–7
Wright MR, Davies NM, Jamali F. Rationale for the development of stereochemically pure enantiomers: are the R enantiomers of NSAIDs inactive? J Pharm Sci 1994; 83: 911–2
Zapatero MI, Fernandez MF, Cabre F, et al. Gastrointestinal ulcerogenic effect of racemic ketoprofen and its enantiomers in the rat. V Congreso de Ciencias Farmaceuticas. Alcala de Henares, 15-18 November, 1995
Hersh EV. The efficacy and safety of ketoprofen in postsurgical dental pain. Compend Contin Educ Dent 1991; 12: 234–6
Lobo R, Gallardo F, Henríquez E, et al. Analgesic activity of ketoprofen in post-operative oral surgery pain. IRCS Med Sci 1983; 11: 639–40
Cooper SA, Gelb SB, Cavaliere MBM, et al. An analgesic relative potency assay comparing ketoprofen and aspirin in postoperative dental pain. Adv Ther 1984; 1: 410–8
Mehlisch D, Frakes L, Cavaliere MB, et al. Double-blind parallel comparison of single oral doses of ketoprofen, codeine, and placebo in patients with moderate to severe dental pain. J Clin Pharmacol 1984; 24: 486–92
Cooper SA, Berrie R, Cohn P. Comparison of ketoprofen, ibuprofen, and placebo in a dental surgery pain model. Adv Ther 1988; 5: 43–53
Niemi L, Tuominen M, Pitkanen M, et al. Comparison of parenteral diclofenac and ketoprofen for postoperative pain relief after maxillofacial surgery. Acta Anaesthesiol Scand 1995; 39: 96–9
Fernández MF, Calvo L, Ferrer X, et al. Study of oral analgesia in mice in the test of phenylbenzoquinone-induced abdominal pain. 1993. Report code: PHAR12/LM-1158.TRIS/93
Gay C, Planas E, Donado M, et al. Analgesic effect of low doses of dexketoprofen in the dental pain model: a randomised, double-blind, placebo-controlled study. Clin Drug Invest 1996; 11: 320–30
Li G, Treiber G, Maier K, et al. Disposition of ibuprofen in patients with liver cirrhosis. Stereochemical considerations. Clin Pharmacokinet 1993; 25: 154–63
Castillo M, Smith PC. Disposition and reactivity of ibuprofen and ibufenac acyl glucuronides in vivo in the Rhesus monkey and in vitro with human serum albumin. Drug Metab Dispos 1995; 23: 566–72
Castillo M, Lam YWF, Dooley MA, et al. Disposition and covalent binding of ibuprofen and its acyl glucuronide in the elderly. Clin Pharmacol Ther 1995; 57: 636–44
Smith PC, Liu JH. Covalent binding of suprofen to renal tissue of rat correlates with excretion of its acyl glucuronide. Xenobiotica 1995; 25: 531–40
Rudy AC, Knight PM, Brater DC, et al. Enantioselective disposition of ibuprofen in elderly persons with and without renal impairment. J Pharm Exp Ther 1995; 273: 88–93
Chen CY, Chen CS. Stereoselective disposition of ibuprofen in patients with compromised renal haemodynamics. Br J Clin Pharmacol 1995; 40: 67–72
Xiaotao Q, Hall SD. Enantioselective effects of experimental diabetes mellitus on the metabolism of ibuprofen. J Pharm Exp Ther 1995; 274: 1192–8
Rey E, Pariente-Khayat A, Gouyet L, et al. Stereoselective disposition of ibuprofen enantiomers in infants. Br J Clin Pharmacol 1994; 38: 373–5
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mauleón, D., Artigas, R., García, M.L. et al. Preclinical and Clinical Development of Dexketoprofen. Drugs 52 (Suppl 5), 24–46 (1996). https://doi.org/10.2165/00003495-199600525-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-199600525-00005