Summary
Synopsis
Sulconazole is a substituted imidazole antimicrobial agent structurally related to other drugs in this group. It possesses a broad spectrum of activity in vitro against dermato-phytes, yeasts and some Grampositive bacteria. The efficacy and safety of sulconazole 1% cream has been demonstrated in controlled clinical studies in patients with superficial dermatophyte or yeast infections. In these trials, sulconazole generally displayed similar efficacy to clotrimazole, econazole and miconazole, although in a few studies sulconazole produced better and/or quicker improvement than clotrimazole or miconazole in small numbers of patients with tinea pedis. Further studies in larger groups of patients are needed to confirm these encouraging preliminary findings.
Thus, sulconazole is an effective and well tolerated alternative to other topical imidazole drugs in the treatment of superficial fungal infections of the skin.
Pharmacodynamic Properties
Sulconazole possesses a broad spectrum of antifungal activity, inhibiting the growth of dermatophytes, yeasts and various filamentous and dimorphic fungi at concentrations below 5 mg/L in vitro. To overcome inconsistencies in MIC values caused by varying experimental conditions, the determination of relative inhibition factors (RIF) of antifungal drugs has been proposed. It has been shown that against representatives of pathogenic yeasts, dermatophytes and Aspergilli, the RIF values of sulconazole were broadly similar to those of other imidazoles. The fungicidal potency of sulconazole in vitro depends on its concentration and on the growth phase of the inoculum cells. Sulconazole has also demonstrated antibacterial activity in vitro, with MIC values below 12.5 mg/L, against several Staphylococcus species, Streptococcus faecalis and certain Grampositive anaerobes.
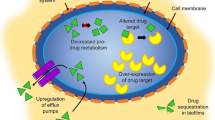
It appears that sulconazole exerts antifungal activity through effects which destroy the capacity of the fungal cell membrane to maintain the intracellular environment.
Pharmacokinetic Properties
About 12% of a topically administered (forearm) dose of sulconazole 1% cream was estimated to be percutaneously absorbed in humans. This value varied markedly in different animal species.
Therapeutic Use
In controlled, comparative clinical trials of patients with superficial fungal infections of the skin, sulconazole has demonstrated clear superiority over placebo and was generally of similar overall clinical efficacy to clotrimazole, econazole and miconazole. Treatment was normally administered twice daily for between 2 and 5 weeks in these studies. Two studies showed that compared with clotrimazole, sulconazole possessed significantly greater overall clinical efficacy in patients with tinea cruris or tinea pedis, and it appears that sulconazole may relieve some of the symptoms of tinea pedis (erythema, scaling, pruritus and maceration/erosion) more quickly than clotrimazole. The 2 drugs were otherwise found to be of similar efficacy, with mycological cure achieved in over 70% of patients. No significant differences were reported in the results of 2 comparisons with econazole, both drugs achieving very high cure rates in patients with tinea pedis or tinea cruris. Likewise, sulconazole and miconazole were generally of similar overall clinical efficacy, although in 2 studies of patients with tinea pedis sulconazole tended towards superiority, but the differences were not statistically significant. Sulconazole again tended to cause a more rapid relief of some clinical symptoms.
Reported relapse rates 4 to 12 weeks following the end of treatment were low for sulconazole and the other imidazole antifungal drugs tested. Differences that were observed usually favoured sulconazole.
Side Effects
Sulconazole has generally been very well tolerated in clinical trials. In the largest study, involving 323 patients, the overall incidence rate of adverse effects was 3.4%, with redness, irritation, contact dermatitis and pruritus being the most frequently reported. Few patients have withdrawn from sulconazole treatment due to side effects.
Administration
Sulconazole 1% cream should be rubbed gently into the affected and surrounding skin area twice daily. To minimise the risk of reinfection treatment should continue for 3 weeks in Candida infections, tinea cruris, tinea corporis and pityriasis versicolor, and for 4 weeks in patients with tinea pedis.
Similar content being viewed by others
References
Avila JM. Treatment of dermatomycoses with sulconazole 1% nitrate cream or miconazole nitrate 2% cream. Current Therapeutic Research 38: 328–333, 1985
Beggs WH. The effect of antifungal imidazoles on resting cells of Candida parapsilosis. IRCS Medical Science: Biochemistry 11: 677, 1983
Beggs WH. Influence of growth phase on the susceptibility of Candida albicans to butoconazole, oxiconazole, and sulconazole. Journal of Antimicrobial Chemotherapy 16: 397–399, 1985
Clissold SP, Heel RC. Tioconazole: a review of its antimicrobial activity and therapeutic use in superficial mycoses. Drugs 31: 29–51, 1986
Fujihara M, Hirakoso K, Harigaya S. Pharmacokinetics of sulconazole nitrate. (1) Fate in rats after application to the skin. Pharmacometrics 28: 145–154, 1984
Gip L, Forsström S. A double-blind parallel study of sulconazole nitrate 1% cream compared with miconazole nitrate 2% cream in dermatophytoses. Mykosen 26: 231–241, 1983
Heel RC, Brogden RN, Speight TM, A very GS. Econazole: a review of its antifungal activity and therapeutic efficacy. Drugs 16: 177–201, 1978
Iwata K, Yamamoto Y. Studies on the antifungal activities of sulconazole nitrate. I. In vitro antifungal activities. Japanese Journal of Medical Mycology 23: 314–317, 1982
Iwata K, Yamamoto Y. Studies on the antifungal activities of sulconazole nitrate. II. Influences of various factors on the antifungal activity. Japanese Journal of Medical Mycology 25: 147–157, 1984
Kerridge D. Mode of action of clinically important antifungal drugs. In Rose & Tempest (Eds) Advances in microbial physiology, Vol. 27, pp. 1–72, Academic Press, London, 1986
Lassus A, Forsström S. A double-blind parallel study comparing sulconazole with econazole in the treatment of dermatophytoses. Mykosen 27: 592–598, 1984
Lassus A, Forsström S, Salo O. A double-blind comparison of sulconazole nitrate 1% cream with clotrimazole 1% cream in the treatment of dermatophytoses. British Journal of Dermatology 108: 195–198, 1983
McVie DH, Littlewood S, Allen BR, Pollock AC, Wood P, et al. Sulconazole versus clotrimazole in the treatment of dermatophytosis. Clinical and Experimental Dermatology 11: 613–618, 1986
Nishizawa Y, Ozeki S, Takahashi N. Clinical study of RS 44872 (sulconazole nitrate) solution in dermatomycosis. Japanese Journal of Clinical and Experimental Medicine 61: 3401–3404, 1984
Odds FC. Laboratory evaluation of antifungal agents: a comparative study of five imidazole derivatives of clinical importance. Journal of Antimicrobial Chemotherapy 6: 749–761, 1980
Odds FC, Abbott AB. Relative inhibition factors: a novel approach to the assessment of antifungal antibiotics in vitro. Journal of Antimicrobial Chemotherapy 13: 31–43, 1984
Odds FC, Webster CE, Abbott AB. Antifungal relative inhibition factors: BAY 1-9139, bifonazole, butoconazole, isoconazole, itraconazole (R51211), oxiconazole, Ro 14-4767/002, sulconazole, terconazole and vibrinazole (BAY n-7133) compared in vitro with nine established antifungal agents. Journal of Antimicrobial Chemotherapy 14: 105–114, 1984
Qadripur S-A. Double-blind parallel comparison of sulconazole nitrate, 1% cream and powder with econazole, 1% cream and powder, in the treatment of cutaneous dermatophytoses. Current Therapeutic Research 35: 753–758, 1984
Rajan VS, Thirumoorthy T. Treatment of cutaneous candidiasis: a double blind parallel comparison of sulconazole nitrate 1% cream and clotrimazole 1% cream. Australian Journal of Dermatology 24: 33–36, 1983
RS 44872 Study Group. Clinical evaluation of RS 44872 (sulconazole nitrate) cream in dermatomycosis. Nishinihon Journal of Dermatology 46: 769–782, 1984
Sawyer PR, Brogden RN, Pinder RM, Speight TM, Avery GS. Miconazole: a review of its antifungal activity and therapeutic efficacy. Drugs 9: 406–423, 1975a
Sawyer PR, Brogden RN, Pinder RM, Speight TM, Avery GS. Clotrimazole: a review of its antifungal activity and therapeutic efficacy. Drugs 9: 424–447, 1975b
Sud IJ, Chou D-L, Feingold DS. Effect of free fatty acids on liposome susceptibility to imidazole antifungals. Antimicrobial Agents and Chemotherapy 16: 660–663, 1979
Tanenbaum L, Anderson C, Rosenberg MJ, Akers W. 1% sulconazole cream v 2% miconazole cream in the treatment of tinea versicolor. Archives of Dermatology 120: 216–219, 1984
Tanenbaum L, Anderson C, Rosenberg M, Dorr A. A new treatment for cutaneous candidiasis: sulconazole nitrate cream 1%. International Journal of Dermatology 22: 318–320, 1983
Tanenbaum L, Anderson C, Rosenberg MJ, Howard W, Mc-Daniel W, et al. Sulconazole nitrate 1% cream: a comparison with miconazole in the treatment of tinea pedis and tinea cruris/ corporis. Cutis 30: 105–118, 1982
Watanabe S, Shimozuma M, Hei K, Kukita A. Clinical evaluation of RS 44872 (sulconazole nitrate) solution in the treatment of dermatomycosis. Nishinihon Journal of Dermatology 46: 966–972, 1984
Woscoff A, Carabeli S. Treatment of tinea pedis with sulconazole nitrate 1% cream or miconazole nitrate 2% cream. Current Therapeutic Research 39: 753–757, 1986
Yamaguchi H, Hiratani T, Iwata K. Studies on the mode of action of a new imidazole antimycotic sulconazole. Japanese Journal of Medical Mycology 24: 253–262, 1983
Yoshida H, Kasuga O, Yamaguchi T. Studies on antifungal activities of sulconazole. I. In vitro antimicrobial activity. Chemotherapy 32: 477–484, 1984
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Benfield, P., Stephen, P. Sulconazole. Drugs 35, 143–153 (1988). https://doi.org/10.2165/00003495-198835020-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-198835020-00004