Abstract
Although several routes of administration can be considered for new drug entities, the oral route remains the most popular. To predict the in vivo performance of a drug after oral administration from in vitro data, it is essential that the factors limiting absorption can be modelled. Factors limiting oral drug absorption are typically slow and/or incomplete dissolution, formation of insoluble complexes and/or decomposition in the gastrointestinal lumen, poor net permeability and first-pass metabolism. Although many attempts have been made to make global forecasts of oral bioavailability based on a single parameter (ranging from the partition coefficient [logP] to the polar surface area), it is clear from the diversity of properties that can influence delivery of drugs via the oral route that such an approach can at best lead to a qualitative estimation. To predict in vivo performance in a more quantitative way, it is instead necessary to identify the extent to which each of the aforementioned factors can limit absorption, and then combine the information into a comprehensive model of the absorptive processes. Much progress has been made in the last 10 years on developing methods to pin down the extent to which each of the factors actually limits the absorption of a given compound and, concomitantly, physiological models have been evolved, which show promise in terms of being able to integrate the information generated about each of the individual limiting factors. This article attempts to summarize recent progress on the various fronts as a kind of ‘progress report’ towards quantitative prediction of oral drug absorption.













Similar content being viewed by others
References
Dressman JB, Reppas C. In vitro-in vivo correlations for lipophilic, poorly water-soluble drugs. Eur J Pharm Sci 2000; 11 Suppl. 2: S73–80
Dressman JB, Fleisher D. Mixing-tank model for predicting dissolution rate control or oral absorption. J Pharm Sci 1986; 75(2): 109–16
US FDA Center for Evaluation and Research. Guidance for industry: waiver of in vivo bioavailability and bioequivalence studies for immediate-release solid oral dosage forms based on a biopharmaceutics classification system [online]. Available from URL: http://www.fda.gov/cder/guidance/3618fnl.pdf [Accessed 2008 Feb 29]
Dressman JB, Amidon GL, Reppas C, et al. Dissolution testing as a prognostic tool for oral drug absorption: immediate release dosage forms. Pharm Res 1998; 15(1): 11–22
Jantratid E, Janssen N, Reppas C, et al. Dissolution media simulating conditions in the proximal human gastrointestinal tract: an update. Pharm Res 2008; 25(7): 1663–76
Vertzoni M, Dressman J, Butler J, et al. Simulation of fasting gastric conditions and its importance for the in vivo dissolution of lipophilic compounds. Eur J Pharm Biopharm 2005; 60(3): 413–7
Finholt P, Solvang S. Dissolution kinetics of drugs in human gastric juice: the role of surface tension. J Pharm Sci 1968; 57(8): 1322–6
Macheras P, Koupparis M, Apostolleli E. Dissolution of 4 controlled-release theophylline formulations in milk. Int J Pharm 1987; 36: 73–9
Junginger HE, Verhoeven J, Peschier LJC. A new in vitro model to detect interactions between controlled release dosage forms and food. Acta Pharm Technol 1990; 36(3): 155–60
Krämer J. Korrelation biopharmazeutischer in vivo und in vitro Daten von Theophyllin und Verapamil Retardpräparaten [PhD dissertation]. Heidelberg: Ruprecht-Karls-University, 1995
Dermentzoglou LC. Changes in upper gastrointestinal pH with aging: implications for drug absorption [PhD dissertation]. Ann Arbor (MI): University of Michigan, 1989
Lindahl A, Ungell AL, Knutson L, et al. Characterization of fluids from the stomach and proximal jejunum in men and women. Pharm Res 1997; 14(4): 497–502
Kalantzi L, Goumas K, Kalioras V, et al. Characterization of the human upper gastrointestinal contents under conditions simulating bioavailability/bioequivalence studies. Pharm Res 2006; 23(1): 165–76
Fordtran JS, Locklear TW. Ionic constituents and osmolality of gastric and small-intestinal fluids after eating. Am J Dig Dis 1966; 11(7): 503–21
Welling PG. Effects of food on drug absorption. Annu Rev Nutr 1996; 16: 383–415
Custodio JM, Wu C-Y, Benet LZ. Predicting drug disposition, absorption/elimination/transporter interplay and the role of food on drug absorption. Adv Drug Deliv Rev 2008; 60: 717–33
Takano R, Sugano K, Higashida A, et al. Oral absorption of poorly water-soluble drugs: computer simulation of fraction absorbed in humans from a miniscale dissolution test. Pharm Res 2006; 23(6): 1144–56
Zangenberg NH, Müllertz A, Kristensen HG, et al. A dynamic in vitro lipolysis model: I. Controlling the rate of lipolysis by continuous addition of calcium. Eur J Pharm Sci 2001; 14(2): 115–22
Zangenberg NH, Müllertz A, Kristensen HG, et al. A dynamic in vitro lipolysis model: II. Evaluation of the model. Eur J Pharm Sci 2001; 14(3): 237–44
Fatouros DG, Müllertz A. Using in vitro dynamic lipolysis modeling as a tool for exploring IVIVC relationships for oral lipid-based formulations. In: Hauss DJ, editor. Oral lipid-based formulations: enhancing the bioavailability of poorly water-soluble drugs. 1st ed. New York: Informa Healthcare USA, Inc., 2007: 257–72
Conners K, Amidon GL, Valentino JS. Chemical stability of pharmaceuticals: a handbook for pharmacists. 2nd ed. New York: Wiley & Sons, Inc., 1986
Becker C, Dressman JB, Amidon GL, et al. Biowaiver monographs for immediate release solid oral dosage forms: isoniazid. J Pharm Sci 2007; 96(3): 522–31
Winiwarter S, Bonham NM, Ax F, et al. Correlation of human jejunal permeability (in vivo) of drugs with experimentally and theoretically derived parameters: a multivariate data analysis approach. J Med Chem 1998; 41(25): 4939–49
Lennernäs H. Human intestinal permeability. J Pharm Sci 1998; 87(4): 403–10
Wohnsland F, Faller B. High-throughput permeability pH profile and high-throughput alkane/water log P with artificial membranes. J Med Chem 2001; 44(6): 923–30
Kansy M, Senner F, Gubernator K. Physicochemical high throughput screening: parallel artificial membrane permeation assay in the description of passive absorption processes. J Med Chem 1998; 41(7): 1007–10
Sugano K, Hamada H, Machida M, et al. High throughput prediction of oral absorption: improvement of the composition of the lipid solution used in parallel artificial membrane permeation assay. J Biomol Screen 2001; 6(3): 189–96
Zhu C, Jiang L, Chen TM, et al. A comparative study of artificial membrane permeability assay for high throughput profiling of drug absorption potential. Eur J Med Chem 2002; 37(5): 399–407
Avdeef A. The rise of PAMPA. Expert Opin Drug Metab Toxicol 2005; 1(2): 325–42
Avdeef A, Bendels S, Di L, et al. PAMPA-critical factors for better predictions of absorption. J Pharm Sci 2007; 96(11): 2893–909
Hidalgo IJ, Raub TJ, Borchardt RT. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 1989; 96(3): 736–49
Artursson P, Palm K, Luthman K. Caco-2 monolayers in experimental and theoretical predictions of drug transport. Adv Drug Deliv Rev 2001; 46(1–3): 27–43
Alsenz J, Haenel E. Development of a 7-day, 96-well Caco-2 permeability assay with high-throughput direct UV compound analysis. Pharm Res 2003; 20(12): 1961–9
Ingels F, Beck B, Oth M, et al. Effect of simulated intestinal fluid on drug permeability estimation across Caco-2 monolayers. Int J Pharm 2004; 274(1–2): 221–32
Stewart BH, Chan OH, Lu RH, et al. Comparison of intestinal permeabilities determined in multiple in vitro and in situ models: relationship to absorption in humans. Pharm Res 1995; 12(5): 693–9
Chong S, Dando SA, Soucek KM, et al. In vitro permeability through Caco-2 cells is not quantitatively predictive of in vivo absorption for peptide-like drugs absorbed via the dipeptide transporter system. Pharm Res 1996; 13(1): 120–3
Lennernäs H, Palm K, Fagerholm U, et al. Comparison between active and passive transport in human intestinal epithelial (Caco-2) cells in vitro and human jejunum in vivo. Int J Pharm 1996; 127: 103–7
Yee S. In vitro permeability across Caco-2 cells (colonic) can predict in vivo (small intestinal) absorption in man: fact or myth. Pharm Res 1997; 14(6): 763–6
Collett A, Sims E, Walker D, et al. Comparison of HT29-18-C1 and Caco-2 cell lines as models for studying intestinal paracellular drug absorption. Pharm Res 1996; 13(2): 216–21
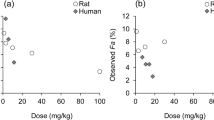
Chiou WL, Barve A. Linear correlation of the fraction of oral dose absorbed of 64 drugs between humans and rats. Pharm Res 1998; 15(11): 1792–5
Chiou WL, Buehler PW. Comparison of oral absorption and bioavailablity of drugs between monkey and human. Pharm Res 2002; 19(6): 868–74
Willmann S, Edginton AN, Dressman JB. Development and validation of a physiology-based model for the prediction of oral absorption in monkeys. Pharm Res 2007; 24(7): 1275–82
Lane JS, Whang EE, Rigberg DA, et al. Paracellular glucose transport plays a minor role in the unanesthetized dog. Am J Physiol 1999; 276 (3 Pt 1): G789–94
He YL, Murby S, Warhurst G, et al. Species differences in size discrimination in the paracellular pathway reflected by oral bioavailability of poly(ethylene glycol) and D-peptides. J Pharm Sci 1998; 87(5): 626–33
Guengerich FP. Characterization of human cytochrome P450 enzymes. FASEB J 1992; 6(2): 745–8
Smith MT, Eadie MJ, Brophy TO. The pharmacokinetics of midazolam in man. Eur J Clin Pharmacol 1981; 19(4): 271–8
Edgar B, Regardh CG, Johnsson G, et al. Felodipine kinetics in healthy men. Clin Pharmacol Ther 1985; 38(2): 205–11
Kleinbloesem CH, van Brummelen P, van de Linde JA, et al. Nifedipine: kinetics and dynamics in healthy subjects. Clin Pharmacol Ther 1984; 35(6): 742–9
Fahr A. Cyclosporin clinical pharmacokinetics. Clin Pharmacokinet 1993; 24(6): 472–95
Roberts CJ. Clinical pharmacokinetics of ranitidine. Clin Pharmacokinet 1984; 9(3): 211–21
Gerkin R, Curry SC, Vance MV, et al. First-order elimination kinetics following baclofen overdose. Ann Emerg Med 1986; 15(7): 843–6
Manley HJ, Bailie GR, McClaran ML, et al. Gentamicin pharmacokinetics during slow daily home hemodialysis. Kidney Int 2003; 63(3): 1072–8
Beermann B, Groschinsky-Grind M. Pharmacokinetics of hydrochlorothiazide in man. Eur J Clin Pharmacol 1977; 12(4): 297–303
Barnwell SG, Laudanski T, Story MJ, et al. Improved oral bioavailability of propranolol in healthy human volunteers using a liver bypass drug delivery system containing oleic acid. Int J Pharm 1992; 88: 423–32
Shimada T, Yamazaki H, Mimura M, et al. Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: studies with liver microsomes of 30 Japanese and 30 Caucasians. J Pharmacol Exp Ther 1994; 270(1): 414–23
Paine MF, Hart HL, Ludington SS, et al. The human intestinal cytochrome P450 ‘pie’. Drug Metab Dispos 2006; 34(5): 880–6
Watkins PB, Wrighton SA, Schuetz EG, et al. Identification of glucocorticoid-inducible cytochromes P-450 in the intestinal mucosa of rats and man. J Clin Invest 1987; 80(4): 1029–36
Paine MF, Khalighi M, Fisher JM, et al. Characterization of interintestinal and intraintestinal variations in human CYP3A-dependent metabolism. J Pharmacol Exp Ther 1997; 283(3): 1552–62
Yang J, Tucker GT, Rostami-Hodjegan A. Cytochrome P450 3A expression and activity in the human small intestine. Clin Pharmacol Ther 2004; 76(4): 391
Kolars JC, Awni WM, Merion RM, et al. First-pass metabolism of cyclosporin by the gut. Lancet 1991; 338(8781): 1488–90
Paine MF, Shen DD, Kunze KL, et al. First-pass metabolism of midazolam by the human intestine. Clin Pharmacol Ther 1996; 60(1): 14–24
von Richter O, Greiner B, Fromm MF, et al. Determination of in vivo absorption, metabolism, and transport of drugs by the human intestinal wall and liver with a novel perfusion technique. Clin Pharmacol Ther 2001; 70(3): 217–27
Lasker JM, Wester MR, Aramsombatdee E, et al. Characterization of CYP2C19 and CYP2C9 from human liver: respective roles in microsomal tolbutamide, S-mephenytoin, and omeprazole hydroxylations. Arch Biochem Biophys 1998; 353(1): 16–28
Galetin A, Houston JB. Intestinal and hepatic metabolic activity of five cytochrome P450 enzymes: impact on prediction of first-pass metabolism. J Pharmacol Exp Ther 2006; 318(3): 1220–9
Zhang QY, Dunbar D, Ostrowska A, et al. Characterization of human small intestinal cytochromes P-450. Drug Metab Dispos 1999; 27(7): 804–9
Peters WH, Kremers PG. Cytochromes P-450 in the intestinal mucosa of man. Biochem Pharmacol 1989; 38(9): 1535–8
Lin JH, Chiba M, Baillie TA. Is the role of the small intestine in first-pass metabolism overemphasized? Pharmacol Rev 1999; 51(2): 135–58
Gibbs MA, Thummel KE, Shen DD, et al. Inhibition of cytochrome P-450 3A (CYP3A) in human intestinal and liver microsomes: comparison of Ki values and impact of CYP3A5 expression. Drug Metab Dispos 1999; 27(2): 180–7
Holtbecker N, Fromm MF, Kroemer HK, et al. The nifedipine-rifampin interaction: evidence for induction of gut wall metabolism. Drug Metab Dispos 1996; 24(10): 1121–3
Fagerholm U. Prediction of human pharmacokinetics-gut-wall metabolism. J Pharm Pharmacol 2007; 59(10): 1335–43
Yang J, Jamei M, Yeo KR, et al. Prediction of intestinal first-pass drug metabolism. Curr Drug Metab 2007; 8(7): 676–84
Van de Waterbeemd H. From in vivo to in vitro/in silico ADME: progress and challenges. Expert Opin Drug Metab Toxicol 2005; 1(1): 1–4
Willmann S, Lippert J, Schmitt W. From physicochemistry to absorption and distribution: predictive mechanistic modelling and computational tools. Expert Opin Drug Metab Toxicol 2005; 1(1): 159–68
Willmann S, Schmitt W, Keldenich J, et al. A physiological model for the estimation of the fraction dose absorbed in humans. J Med Chem 2004; 47(16): 4022–31
Grass GM. Simulation models to predict oral drug absorption from in vitro data. Adv Drug Deliv Rev 1997; 23: 199–219
Butina D, Segall MD, Frankcombe K. Predicting ADME properties in silico: methods and models. Drug Discov Today 2002; 7 Suppl. 11: S83–8
Grass GM, Sinko PJ. Physiologically-based pharmacokinetic simulation modelling. Adv Drug Deliv Rev 2002; 54(3): 433–51
Norris DA, Leesman GD, Sinko PJ, et al. Development of predictive pharmacokinetic simulation models for drug discovery. J Control Release 2000; 65(1–2): 55–62
Obata K, Sugano K, Saitoh R, et al. Prediction of oral drug absorption in humans by theoretical passive absorption model. Int J Pharm 2005; 293(1–2): 183–92
Dickens M, van de Waterbeemd H. Simulation models for drug disposition and drug interactions. Drug Discov Today Biosilico 2004; 2(1): 38–45
Willmann S, Hohn K, Edginton A, et al. Development of a physiology-based whole-body population model for assessing the influence of individual variability on the pharmacokinetics of drugs. J Pharmacokinet Pharmacodyn 2007; 34(3): 401–31
Agoram B, Woltosz WS, Bolger MB. Predicting the impact of physiological and biochemical processes on oral drug bioavailability. Adv Drug Deliv Rev 2001; 50 Suppl. 1: S41–67
Yu LX, Amidon GL. Characterization of small intestinal transit time distribution in humans. Int J Pharm 1998; 171: 157–63
Yu LX, Amidon GL. A compartmental absorption and transit model for estimating oral drug absorption. Int J Pharm 1999; 186(2): 119–25
De Buck SS, Sinha VK, Fenu LA, et al. Prediction of human pharmacokinetics using physiologically based modeling: a retrospective analysis of 26 clinically tested drugs. Drug Metab Dispos 2007; 35(10): 1766–80
Parrott N, Lave T. Prediction of intestinal absorption: comparative assessment of GastroPlus and Idea. Eur J Pharm Sci 2002; 17(1–2): 51–61
Kuentz M, Nick S, Parrott N, et al. A strategy for preclinical formulation development using GastroPlus as pharmacokinetic simulation tool and a statistical screening design applied to a dog study. Eur J Pharm Sci 2006; 27(1): 91–9
Jones HM, Parrott N, Ohlenbusch G, et al. Predicting pharmacokinetic food effects using biorelevant solubility media and physiologically based modelling. Clin Pharmacokinet 2006; 45(12): 1213–26
Schmitt W, Willmann S. Physiology-based pharmacokinetic modeling: ready to be used. Drug Discov Today Technologies 2005; 2(1): 125–32
Willmann S, Lippert J, Sevestre M, et al. PK-Sim®: a physiologically based pharmacokinetic ‘whole-body’ model. Biosilico 2003; 1(4): 121–4
Kleine-Besten M, Willmann S, Edginton AN, et al. The prediction of Cimetidine absorption profiles with PK-SIM® using in vitro dissolution data. German Chapter Annual Meeting of the Controlled Release Society; 2006 February 23–24; Jena
Jantratid E, Prakongpan S, Amidon GL, et al. Feasibility of biowaiver extension to biopharmaceutics classification system class III drug products: Cimetidine. Clin Pharmacokinet 2006; 45(4): 385–99
Blank K, Becker C, Lippert J, et al. Physiology-based models to simulate gut wall metabolism of nifedipine. DPhG Jahrestagung; 2007 October 10–13; Erlangen
Blank K, Becker C, Jantratid E, et al. PBPK modelling to demonstrate the impact of variability in CYP3A-mediated metabolism of nifedipine in different subpopulations. 6th World Meeting on Pharmaceutics, Biopharmaceutics and Pharmaceutical Technology; 2008 April 7–10; Barcelona
Bode H, Brendel E, Ahr G, et al. Investigation of nifedipine absorption in different regions of the human gastrointestinal (GI) tract after simultaneous administration of 13C- and 12C-nifedipine. Eur J Clin Pharmacol 1996; 50(3): 195–201
Guengerich FP, Martin MV, Beaune PH, et al. Characterization of rat and human liver microsomal cytochrome P-450 forms involved in nifedipine oxidation, a prototype for genetic polymorphism in oxidative drug metabolism. J Biol Chem 1986; 261(11): 5051–60
Acknowledgements
Kirsten Thelan is financially supported by Bayer Technology Services GmbH (Leverkusen, Germany) and Ekarat Jantratid by F. Hoffmann La Roche (Nutley, NJ, USA). No sources of funding were used to assist in the preparation of this review. The authors have no conflicts of interest that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dressman, J.B., Thelen, K. & Jantratid, E. Towards Quantitative Prediction of Oral Drug Absorption. Clin Pharmacokinet 47, 655–667 (2008). https://doi.org/10.2165/00003088-200847100-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-200847100-00003