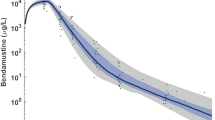
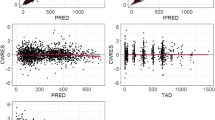
Abstract
Background and objective
The BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine and prednisone) chemotherapy regimen for the treatment of advanced Hodgkin’s lymphoma has a superior outcome, but its toxicity (mainly haematotoxicity) is pronounced and highly variable. The present study was conducted to address the role of pharmacokinetics in individual toxicity.
Study design
Three plasma samples and a 24-hour urine collection for day 1 of the first three cycles of chemotherapy were analysed in 30 patients, and the pharmacokinetic parameters of the respective drugs were estimated by population pharmacokinetic methods (nonlinear mixed-effects model [NONMEM] software). Demographic data, doses and durations of infusion were also recorded. The effect of these parameters on platelet counts was estimated by analysis of covariance using a general linear model.
Results
The pharmacokinetic parameters and respective covariates were similar to the published data. The body surface area, peak concentrations of etoposide, urinary recovery of dechloroethylcyclophosphamide (formed by cytochrome P450 [CYP] 3A4) relative to the cyclophosphamide dose and number of cycles had a significant effect on toxicity. These factors explained 37% of the interindividual variability in the change in platelet counts from day 1 to day 8 of each cycle.
Conclusion
The results show that the individual pharmacokinetics of BEACOPP drugs are an important link between dosage and toxicity. Accordingly, individualisation of treatment based on pharmacokinetics may result in more uniform toxicity. Individualisation may also allow escalation of the mean dose, which is probably related to better efficacy. As a consequence of the present study, infusion rates should be standardised, and the potential of a dose reduction in the first cycle and of CYP3A4 phenotyping should be addressed in clinical studies.










Similar content being viewed by others
References
Diehl V, Sieber M, Ruffer U, et al. BEACOPP: an intensified chemotherapy regimen in advanced Hodgkin’s disease. Ann Oncol 1997; 8: 143–8
Diehl V, Franklin J, Pfreundschuh M, et al. Standard and increased-dose BEACOPP chemotherapy compared with COPP-ABVD for advanced Hodgkin’s disease. N Engl J Med 2003; 348: 2386–95
Engel C, Loeffler M, Schmitz S, et al. Acute hematologic toxicity and practicability of dose-intensified BEACOPP chemotherapy for advanced stage Hodgkin’s disease. German Hodgkin’s Lymphoma Study Group (GHSG). Ann Oncol 2000; 11: 1105–14
Diehl V, Franklin J, Hasenclever D, et al. BEACOPP, a new dose-escalated and accelerated regimen, is at least as effective as COPP/ABVD in patients with advanced-stage Hodgkin’s lymphoma: interim report from a trial of the German Hodgkin’s Lymphoma Study Group. J Clin Oncol 1998; 16: 3810–21
Tesch H, Diehl V, Lathan B, et al. Moderate dose escalation for advanced stage Hodgkin’s disease using the bleomycin, etoposide, adriamycin, cyclophosphamide, vincristine, procarbazine, and prednisone scheme and adjuvant radiotherapy: a study of the German Hodgkin’s Lymphoma Study Group. Blood 1998; 92: 4560–7
Klimm B, Reineke T, Haverkamp H, et al. Role of hematotoxicity and sex in patients with Hodgkin’s lymphoma: an analysis from the German Hodgkin Study Group. J Clin Oncol 2005; 23: 8003–11
Brosteanu O, Hasenclever D, Loeffler M, et al. Low acute hematological toxicity during chemotherapy predicts reduced disease control in advanced Hodgkin’s disease. Ann Hematol 2004; 83: 176–82
Bergh J. Adjuvant chemotherapy for breast cancer: “one fits all”? Breast 2005; 14: 564–9
McLeod HL, Evans WE. Clinical pharmacokinetics and pharmacodynamics of epipodophyllotoxins. Cancer Surv 1993; 17: 253–68
Veal GJ, Coulthard SA, Boddy AV. Chemotherapy individualization. Invest New Drugs 2003; 21: 149–56
Fuhr U, Kirchheiner J. Individual variation in the many steps from dosing to antineoplastic effects. Int J Clin Pharmacol Ther 2005; 43: 573–4
Liliemark J, Peterson C. Pharmacokinetic optimisation of anticancer therapy. Clin Pharmacokinet 1991; 21: 213–31
Sandström M, Lindman H, Nygren P, et al. Population analysis of the pharmacokinetics and the haematological toxicity of the fluorouracil-epirubicin-cyclophosphamide regimen in breast cancer patients. Cancer Chemother Pharmacol 2006; 58: 143–56
Ette EI, Kelman AW, Howie CA, et al. Interpretation of simulation studies for efficient estimation of population pharmacokinetic parameters. Ann Pharmacother 1993; 27: 1034–9
Mentre F, Burtin P, Merle Y, et al. Sparse-sampling optimal designs in pharmacokinetics and toxicokinetics. Drug Information J 1995; 29: 997–1019
Rietbrock S. Optimal sampling designs in studies on population kinetics of antineoplastic agents. Eur J Clin Pharmacol 1997; 52: A15
Hardy RW, Erlichman C, Soldin SJ. High-performance liquid chromatographic measurement of cyclophosphamide in serum. Ther. Drug Monit 1984; 6: 313–8
Hempel G, Schulze-Westhoff P, et al. Therapeutic drug monitoring of doxorubicin in paediatric oncology using capillary electrophoresis. Electrophoresis 1998; 19: 2939–43
Stremetzne S, Jaehde U, Schunack W. Determination of the cytotoxic catechol metabolite of etoposide (3′O-demethyleto-poside) in human plasma by high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl 1997; 703: 209–15
Huber F, Wiedemann M, Heinrich G, et al. Development of a high performance liquid chromatography method for the simultaneous measurement of prednisone and prednisolone. Arzneimittelforschung 1990; 40: 926–31
Kasel D, Jetter A, Harlfinger S, et al. Quantification of cyclophosphamide and its metabolites in urine using liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 2004; 18: 1472–8
Shiba DA, Weinkam RJ. Quantitative analysis of procarbazine, procarbazine metabolites and chemical degradation products with application to pharmacokinetic studies. J Chromatogr B Biomed Sci Appl 1982; 229: 397–407
Kasel D, Jetter A, Wilde S, et al. Urinary procarbazine excretion during the BEACOPP polychemotherapy regimen in patients with Hodgkin’s disease. Eur J Clin Pharmacol 2001; 57: A32
Busse D, Kroemer HK. Dose-dependancy of oxazaphosphorine pharmacokinetics. Int J Clin Pharmacol Ther 1997; 35: 71–2
de Jonge ME, Huitema AD, Rodenhuis S, et al. Clinical pharmacokinetics of cyclophosphamide. Clin Pharmacokinet 2005; 44: 1135–64
Zhang J, Tian Q, Yung Chan S, et al. Metabolism and transport of oxazaphosphorines and the clinical implications. Drug Metab Rev 2005; 37: 611–703
Tofolli G, Corona G, Basso B, et al. Pharmacokinetic optimisation of treatment with oral etoposide. Clin Pharmacokinet 2004; 43: 441–66
Brindley CJ, Antoniw P, Newlands ES, et al. Pharmacokinetics and toxicity of the epipodophyllotoxin derivative etoposide (VP 16–213) in patients with gestational choriocarcinoma and malignant teratoma. Cancer Chemother Pharmacol 1985; 15: 66–71
Speth PA, van Hoesel QG, Haanen C. Clinical pharmacokinetics of doxorubicin. Clin Pharmacokinet 1988; 15: 15–31
Mross K, Maessen P, van der Vijgh WJ, et al. Pharmacokinetics and metabolism of epidoxorubicin and doxorubicin in humans. J Clin Oncol 1988; 6: 517–26
Danesi R, Fogli S, Gennari A, et al. Pharmacokinetic-pharmacodynamic relationships of the anthracycline anticancer drugs. Clin Pharmacokinet 2002; 41: 431–44
Kasel D, Baumhakel M, Fuhr U. Biodegradation of procarbazine by human liver microsomes. Int J Clin Pharmacol Ther 2000; 38: 153–5
Begg EJ, Atkinson HC, Gianarakis N. The pharmacokinetics of corticosteroid agents. Med J Aust 1987; 146: 37–41
Karlsson MO, Sheiner LB. The importance of modelling interoccasion variability in population pharmacokinetics. J Pharmacokinet Biopharm 1993; 21: 735–50
Cockroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16: 31–41
Ishibashi T, Yano Y, Oguma T. Population pharmacokinetics of platinum after nedaplatin administration and model validation in adult patients. Br J Clin Pharmacol 2003; 56: 205–13
Powis G, Reece P, Ahmann DL, et al. Effect of body weight on the pharmacokinetics of cyclophosphamide in breast cancer patients. Cancer Chemother Pharmacol 1987; 20: 219–22
Nguyen L, Chatelut E, Chevreau C, et al. Population pharmacokinetics of total and unbound etoposide. Cancer Chemother Pharmacol 1998; 41: 125–32
Freyer G, Tranchand B, Ligneau B, et al. Population pharmacokinetics of doxorubicin, etoposide and ifosfamide in small cell lung cancer patients: results of a multicentre study. Br J Clin Pharmacol 2000; 50: 315–24
Li J, Gwilt PR. The effect of age on the early disposition of doxorubicin. Cancer Chemother Pharmacol 2003; 51: 395–402
Gurney H: How to calculated the dose of chemotherapy. Br J Cancer 2002; 86: 1297–302
Greco FA, Hainsworth JD: Prolonged administration of low-daily-dose etoposide: a superior dosing schedule? Cancer Chemother Pharmacol 1994; 34 Suppl: S101–4
Clark PI, Slevin ML, Joel SP, et al. A randomized trial of two etoposide schedules in small-cell lung cancer: the influence of pharmacokinetics on efficacy and toxicity. J Clin Oncol 1994; 12: 1427–35
Yule SM, Price L, McMahon AD, et al. Cyclophosphamide metabolism in children with non-Hodgkin’s lymphoma. Clin Cancer Res 2004; 10: 455–60
Ayash LJ, Wright JE, Tretyakov O, et al. Cyclophosphamide pharmacokinetics: correlation with cardiac toxicity and tumor response. J Clin Oncol 1992; 10: 995–1000
Petros WP, Hopkins PJ, Spruill S, et al. Associations between drug metabolism genotype, chemotherapy pharmacokinetics, and overall survival in patients with breast cancer. J Clin Oncol 2005; 23: 6117–25
Xie H, Griskevicius L, Stahle L, et al. Pharmacogenetics of cyclophosphamide in patients with haematological malignancies. Eur J Pharm Sci 2006; 27: 54–61
de Jonge ME, Huitema AD, Tukker AC, et al. Accuracy, feasibility, and clinical impact of prospective Bayesian pharmacokinetically guided dosing of cyclophosphamide, thiotepa, and carboplatin in high-dose chemotherapy. Clin Cancer Res 2005; 11: 273–83
McDonald GB, McCune JS, Batchelder A, et al. Metabolism-based cyclophosphamide dosing for hematopoietic cell transplant. Clin Pharmacol Ther 2005; 78: 298–308
Fuchs M, Franklin J, Klimm B, et al. Mortality during treatment of patients with advanced Hodgkin’s lymphoma undergoing dose escalated BEACOPP chemotherapy: an analysis of the German Hodgkin Study Group (GHSG) [abstract]. Blood 2005; 106: 2668
Zorsky PE, Perkins JB. Optimizing high-dose therapy using pharmacokinetic principles. Semin Oncol 1993; 20 (5 Suppl.6): 2–18
Sandström M, Freijs A, Larsson R, et al. Lack of relationship between systemic exposure for the component drug of the fluorouracil, epirubicin, and 4-hydroxycyclophosphamide regimen in breast cancer patients. J Clin Oncol 1996; 14: 1581–8
Chatelut E, Chevreau C, Blancy E, et al. Pharmacokinetics and toxicity of two modalities of etoposide infusion in metastatic non-small-cell lung carcinoma. Cancer Chemother Pharmacol 1990; 26: 365–8
Bielack SS, Erttmann R, Winkler K, et al. Doxorubicin: effect of different schedules on toxicity and anti-tumor efficacy. Eur J Cancer Clin Oncol 1989; 25: 873–82
Hortobagyi GN. Anthracyclines in the treatment of cancer: an overview. Drugs 1997; 54 Suppl. 4: 1–7
Ploylearmsaeng S, Fuhr U, Jetter A. How may anticancer chemotherapy with 5-fluorouracil be individualized? Clin Pharmacokinet 2006; 45: 567–92
Özdemir V, Kalow W, Tang BK, et al. Evaluation of the genetic component of variability in CYP3A4 activity: a repeated drug administration method. Pharmacogenetics 2000; 10: 373–88
Van den Bongard HJ, Mathot RA, Beihnen JH, et al. Pharmacokinetically guided administration of chemotherapeutic agents. Clin Pharmacokinet 2000; 39: 345–67
Acknowledgements
The study was supported in part by the Deutsche Krebshilfe (Grant #70-2337-Fu I), the Köln Fortune Programme of the Medical Faculty of the University of Cologne, and the W. Doerenkamp Foundation, (Cologne, Germany). We are grateful to the patients who participated in this study despite the burden of their disease, and we gratefully acknowledge the support of the following individuals during the conduct of the study in the various German study centres: Dr Zaigler, Mr Menzel, Department of Pharmacology, University Hospital, University of Cologne, Cologne; Dr Breuer, Dr Bredenfeld, Dr Rüffer, Dr Wolf, Dr Weihrauch, Dr Sieber, Dr Tesch, 1st Department of Internal Medicine, University Hospital, University of Cologne, Cologne; Dr Malchau, Department of Clinical Chemistry, University Hospital, University of Cologne, Cologne; Mrs Nisters-Backes, Mrs Koch, Mrs Remmler, German Hodgkin-Lymphoma Study Group, Cologne; Dr Günther, Med. Klinik, Kreiskrankenhaus Reutlingen, Reutlingen; Dr Haedicke, Med. Klinik II, Städtische Kliniken Braunschweig, Braunschweig; Dr Nischik, Dr Gott, Klinik für Hämatologie, Klinikum Minden, Minden; Dr Franzen, Dr Linnemann, Evangelisches Krankenhaus, Mülheim an der Ruhr; Dr Grunewald, Med. Klinik II, Kliniken St Marien, Amberg; Dr Baumgartner, Mrs Jerusalem, Dr Kleinschmidt, Mr Varvenne, Med. Klinik, Rheinische Friedrich-Wilhelms-Universität, Bonn; Dr Hansen, Med. Klinik, Diakonie-Krankenhaus Schwäbisch-Hall, Schwäbisch-Hall; Dr Eberhard, Med. Klinik, Katharinen-Hospital, Stuttgart; Dr Boissevain, 5. Med. Klinik, Klinikum Nord Nürnberg, Nürnberg; Dr Brase, St Elisabeth-Krankenhaus, Saarlouis; Dr Schwonzen, St Walburga-Krankenhaus, Meschede; Dr Schmitz, Dr Steinmetz, Cologne; Dr Sandner, Med. Klinik, Krankenhaus München-Harlaching, München-Harlaching; Dr Grothaus-Pinke, Klinik für Knochenmarkstransplantation, Idar-Oberstein; Dr Mayer, Thoraxklinik Heidelberg, Heidelberg; Dr Ehrsam, Med. Klinik 1, Universität Dresden, Dresden; Dr Orth, Med. Klinik II, Krankenhaus Nordwest, Frankfurt am Main; Dr Hickel, Med. Klinik V, Universitätsklinik Heidelberg, Heidelberg. The authors have no conflicts of interest that are directly relevant to the content of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wilde, S., Jetter, A., Rietbrock, S. et al. Population Pharmacokinetics of the BEACOPP Polychemotherapy Regimen in Hodgkin’s Lymphoma and its Effect on Myelotoxicity. Clin Pharmacokinet 46, 319–333 (2007). https://doi.org/10.2165/00003088-200746040-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-200746040-00005