Abstract
Objective
To examine morphine metabolite serum concentrations in neonates undergoing venoarterial extra corporeal membrane oxygenation (ECMO) and to quantify clearance differences between these neonates and those subjected to noncardiac major surgery.
Patients and methods
This was an observational study in level III referral centre. Fourteen neonates (<7 days old) undergoing ECMO were included. Morphine and concomitant medications were given by protocol, adapted to the clinical conditions of the neonates. Pharmacokinetic findings were compared with those from a previous study in infants after noncardiac major surgery. Nonlinear mixed-effect modelling was used. Parameter estimates were standardised to a 70kg person using allometric modeling
Results
Morphine-3-glucuronide (M3G) was the predominant metabolite. Formation clearance to M3G at the start of ECMO on day 1 was lower than those in postoperative children, but matured more rapidly. After 10 days formation clearances of M3G in neonates on ECMO equalled those of postoperative children. Higher ECMO flows were associated with reduced formation clearances. Elimination clearances of M3G, but not morphine-6-glucuronide (M6G), were lower in the ECMO neonates; this was attributable to reduced renal clearance. These elimination clearances were correlated positively with ECMO flow and negatively with dopamine dose. Haemofiltration cleared M3G and M6G, but not morphine.
Conclusion
Formation clearance to M3G, the predominant metabolite, is reduced during the first 10 days of ECMO. Elimination clearance of M3G and M6G is related to creatinine clearance. ECMO flow had a small effect on metabolite clearance. Higher flows were associated with decreased formation clearances, possibly reflecting illness severity. Dopamine dose reflected decreased renal clearance.








Similar content being viewed by others
References
Iyer LV, Ho MN, Shinn WM, et al. Glucuronidation of 1′-hydroxyestragole (1′-HE) by human UDP-glucuronosyltrans-ferases UGT2B7 and UGT1A9. Toxicol Sci 2003; 73: 36–43
Green MD, King CD, Mojarrabi B, et al. Glucuronidation of amines and other xenobiotics catalyzed by expressed human UDP-glucuronosyltransferase 1A3. Drug Metab Dispos 1998; 26: 507–12
Frances B, Gout R, Monsarrat B, et al. Further evidence that morphine-6 beta-glucuronide is a more potent opioid agonist than morphine. J Pharmacol Exp Ther 1992; 262: 25–31
Paul D, Standifer KM, Inturrisi CE, et al. Pharmacological characterization of morphine-6 beta-glucuronide, a very potent morphine metabolite. J Pharmacol Exp Ther 1989; 251: 477–83
Smith MT, Watt JA, Cramond T. Morphine-3-glucuronide: a potent antagonist of morphine analgesia. Life Sci 1990; 47: 579–85
Labella FS, Pinsky C, Havlicek V. Morphine derivatives with diminished opiate receptor potency show enhanced central excitatory activity. Brain Res 1979; 174: 263–71
Snead III OC. Opiate-induced seizures: a study of mu and delta specific mechanisms. Exp Neurol 1986; 93: 348–58
Woolf CJ. Intrathecal high dose morphine produces hyperalgesia in the rat. Brain Res 1981; 209: 491–5
Yaksh TL, Harty GJ, Onofrio BM. High dose of spinal morphine produce a nonopiate receptor-mediated hyperesthesia: clinical and theoretic implications. Anesthesiology 1986; 64: 590–7
Shohami E, Evron S, Weinstock M, et al. A new animal model for action myoclonus. Adv Neurol 1986; 43: 545–52
Christrup LL. Morphine metabolites. Acta Anaesthesiol Scand 1997; 41: 116–22
Dagan O, Klein J, Gruenwald C, et al. Preliminary studies of the effects of extracorporeal membrane oxygenator on the disposition of common pediatrie drugs. Ther Drug Monit 1993; 15: 263–6
Dagan O, Klein J, Bohn D, et al. Effects of extracorporeal membrane oxygenation on morphine pharmacokinetics in infants. Crit Care Med 1994; 22: 1099–101
Peters JWB, Anderson BJ, Simons SHP, et al. Morphine Pharmacokinetics during venoarterial extracorporeal membrane oxygenation in neonates. Intensive Care Med 2005; 31: 257–63
Bouwmeester J, Andersen B, Tibboel D, et al. Developmental pharmacokinetics of morphine and metabolites in neonates, infants, and children. Br J Anaesth 2004; 92: 208–17
Anderson B. Disentangling PK-PD in neonates. Arch Dis Child Fetal Neonatal Ed 2004; 89: F3–4
Penson RT, Joel SP, Clark S, et al. Limited phase I study of morphine-3-glucuronide. J Pharm Sci 2001; 90: 1810–6
Hanna MH, Peat SJ, Knibb AA, et al. Disposition of morphine-6-glucuronide and morphine in healthy volunteers. Br J Anaesth 1991; 66: 103–7
Holford NHG. A size standard for pharmacokinetics. Clin Pharmacokinet 1996; 30: 329–32
Verwey-van Wissen CP, Koopman-Kimenai PM, Vree TB. Direct determination of codeine, norcodeine, morphine and normorphine with their corresponding O-glucuronide conjugates by high-performance liquid chromatography with electrochemical detection. J Chromotogr 1991; 570: 309–20
West GB, Brown JH, Enquist BJ. A general model for the origin of allometric scaling laws in biology. Science 1997; 276: 122–6
Peters HP. Chpt 4. Physiological correlates of size. In: Beck E, Birks HJB, Conner EF, editors. The ecological implications of body size. Cambridge: Cambridge University Press, 1983: 48–53
Karalis V, Macheras P. Drug disposition viewed in terms of the fractal volume of distribution. Pharm Res 2002; 19: 696–703
Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16: 31–41
Berkenstadt H, Segal E, Mayan H, et al. The pharmacokinetics of morphine and lidocaine in critically ill patients. Intensive Care Med 1999; 25: 110–2
Lynn A, Nespeca MK, Bratton SL, et al. Clearance of morphine in postoperative infants during intravenous infusion: the influence of age and surgery. Anesth Analg 1998; 86: 958–63
Pokela ML, Olkkola KT, Seppala T, et al. Age-related morphine kinetics in infants. Dev Pharmacol Ther 1993; 20: 26–34
Dagan O, Klein J, Bohn D, et al. Morphine pharmacokinetics in children following cardiac surgery: effects of disease and inotropic support. J Cardiothorac Vase Anesth 1993; 7: 396–8
Hasselstrom J, Sawe J. Morphine pharmacokinetics and metabolism in humans. Enterohepatic cycling and relative contribution of metabolites to active opioid concentrations. Clin Pharmacokinet 1993; 24: 344–54
Van Crugten JT, Sallustio BC, Nation RL, et al. Renal tubular transport of morphine, morphine-6-glucuronide, and morphine-3-glucuronide in the isolated perfused rat kidney. Drug Metab Dispos 1991; 19: 1087–92
Bjornsson TD. Use of serum creatinine concentrations to determine renal function. Clin Pharmacokinet 1979; 4: 200–22
Lynn AM, Nespeca MK, Bratton SL, et al. Intravenous morphine in postoperative infants: intermittent bolus dosing versus targeted continuous infusions. Pain 2000; 88: 89–95
McRorie TI, Lynn AM, Nespeca M, et al. The maturation of morphine clearance and metabolism. Am J Dis Child 1992; 146: 972–6
de Wildt SN, Kearns GL, Leeder JS, et al. Glucuronidation in humans: pharmacogenetic and developmental aspects. Clin Pharmacokinet 1999; 36: 439–52
Oda Y, Mizutani K, Hase I, et al. Fentanyl inhibits metabolism of midazolam: competitive inhibition of CYP3A4 in vitro. Br J Anaesth 1999; 82: 900–3
Geiduschek JM, Lynn AM, Bratton SL, et al. Morphine pharmacokinetics during continuous infusion of morphine sulfate for infants receiving extracorporeal membrane oxygenation. Crit Care Med 1997; 25: 360–4
Acknowledgements
The authors have no financial or other potential conflicts of interest that are relevant to the contents of this paper.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
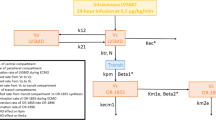
The equations used in the final model were: Equation 8:
Equation 9:
Equation 10:
where FCL03 is the base maturation CLfM3G and FTCL3 is the CLfM3G maturation half-life. Equation 11:
where FCL06 is the base maturation CLfM6G and FTCL6 is the CLfM6G maturation half-life. Equation 12:
Equation 13:
Rights and permissions
About this article
Cite this article
Peters, J.W.B., Anderson, B.J., Simons, S.H.P. et al. Morphine Metabolite Pharmacokinetics during Venoarterial Extra Corporeal Membrane Oxygenation in Neonates. Clin Pharmacokinet 45, 705–714 (2006). https://doi.org/10.2165/00003088-200645070-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-200645070-00005