Abstract
Objectives
To determine the pharmacokinetic parameters of ceftriaxone following an infusion in haemodialysed outpatients and to use these parameters for an optimisation of dosing based on pharmacodynamic indices.
Methods
Fifty haemodialysed patients were enrolled in a single-centre, prospective, open-label study. They received short intravenous infusions of ceftriaxone 1 or 2g every 48 hours for bronchopneumonia immediately after the dialysis session. Total plasma concentrations of ceftriaxone were analysed with a population pharmacokinetic approach using nonlinear mixed-effects modelling. Free drug concentrations were derived from published binding parameters in order to estimate the time when they exceed the minimum inhibitory concentration (MIC).
Results
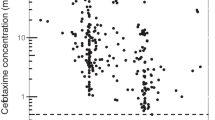
The pharmacokinetics were best described by a two-compartment model. None of the covariates tested (age, bodyweight, height, sex, body mass index, albumin) influenced the pharmacokinetic parameters. The estimated population pharmacokinetic parameters (interindividual variability [percentage of coefficient of variation]) were clearance 0.36 L/h (48%), volume of distribution of the central compartment 4.53L (47%), intercompartmental clearance 10.8 L/h and volume of distribution of the peripheral compartment 9.54L (63%). The terminal elimination half-life (t½β) from plasma was 27.5 hours. The mean (range) times when the free drug concentration exceeded the MIC (T>MIC) following ceftriaxone 1g infusion were 60.3 (53.0–67.7) hours and 2.5 (1.0–3.9) hours for the breakpoints 1 and 8 mg/L (based on free drug concentration), respectively. After administration of ceftriaxone 2g, the T>MIC was 88.5 (78.8–98.3) hours and 17.7 (13.3–22.0) hours for the breakpoints 1 and 8 mg/L, respectively. The simulated free drug concentrations (median, first and third quartile) for 48 and 72 hours following the first dose of ceftriaxone 1g were 1.11, 0.63 and 1.89 mg/L, and 0.63, 0.28 and 1.18 mg/L, respectively. For ceftriaxone 2g infusion, the simulated free concentrations (median, first and third quartile) at 48 and 72 hours were 2.50, 1.40 and 4.52 mg/L, and 1.37, 0.60 and 2.70 mg/L, respectively.
Conclusions
On the basis of decreased clearance in haemodialysed patients, it can be argued that the dose of ceftriaxone should be decreased or the delay between doses should be increased. However, taking into account pharmacodynamic considerations, this study showed that following intravenous administration of ceftriaxone 1g after each dialysis session, some patients were at risk of achieving a concentration below the MIC (1 mg/L), particularly if the second administration occurred 72 hours after the first dosing. Thus, a dose of ceftriaxone 2g intravenously is recommended immediately following dialysis, particularly in patients with severe infections or when the dosing interval will be higher than 48 hours.




Similar content being viewed by others
References
Lamb HM, Ormrod D, Scott LJ, et al. Ceftriaxone: an update of its use in the management of community-acquired and nosocomial infections. Drugs 2002; 62: 1041–89
Sarnak MJ, Jaber BL. Pulmonary infectious mortality among patients with end-stage renal disease. Chest 2001; 120: 1883–7
Fridkin SK. Vancomycin-intermediate and resistant Staphylococcus aureus: what the infectious disease specialist needs to know. Clin Infect Dis 2001; 32: 108–15
Perry TR, Schentag JJ. Clinical use of ceftriaxone: a pharmacokinetic-pharmacodynamic perspective on the impact of minimum inhibitory concentration and serum protein binding. Clin Pharmacokinet 2001; 40: 685–94
Chiu LM, Menhinick AM, Johnson PW, et al. Pharmacokinetics of intravenous azithromycin and ceftriaxone when administered alone and concurrently to healthy volunteers. J Antimicrob Chemother 2002; 50: 1075–9
Gabutti L, Taminelli-Beltraminelli C, Marone C. Clearance of ceftriaxone during haemodialysis using cuprophane, haemophane and polysulfone dialysers. Eur J Clin Pharmacol 1997; 53: 123–6
Patel IH, Sugihara JG, Weinfeld RE, et al. Ceftriaxone pharmacokinetics in patients with various degrees of renal impairment. Antimicrob Agents Chemother 1984; 25: 438–42
Al-Rawithi S, Hussein R, Raines DA, et al. Sensitive assay for the determination of cefazolin or ceftriaxone in plasma utilizing LC. J Pharm Biomed Anal 2000; 22: 281–6
Boeckmann A, Scheiner L, Beal S. NONMEM Users guide: part V. San Francisco (CA): University of California, 1994
Karlsson MO, Jonsson EN, Wiltse CG, et al. Assumption testing in population pharmacokinetic models: illustrated with an analysis of moxonidine data from congestive heart failure patients. J Pharmacokinet Biopharm 1998; 26: 207–46
Ette E. Stability and performance of a population pharmacokinetic model. J Clin Pharmacol 1997; 37: 486–95
Parke J, Holford N, Charles B. A procedure for generating bootstrap samples for the validation of nonlinear mixed-effects population models. Comput Methods Programs Biomed 1999; 59: 19–29
MacNamara PJ, Trueb V, Stoeckel K. Protein binding of ceftriaxone in extravascular fluids. J Pharm Sci 1988; 77: 401–4
Coffey JJ, Bullock FJ, Schoenemann PT. Numerical solution of nonlinear pharmacokinetic equations: effects of plasma protein binding on drug distribution and elimination. J Pharm Sci 1971; 60: 1623–8
Ihara R, Gentleman R. R: a language for data analysis and graphics. J Computational and Graphical Statistics 1996; 5: 299–341
Joynt GM, Lipman J, Gomersall CD, et al. The pharmacokinetics of once-daily dosing of ceftriaxone in critically ill patients. J Antimicrob Chemother 2001; 47: 421–9
Hayton WL, Stoeckel K. Age-associated changes in ceftriaxone pharmacokinetics. Clin Pharmacokinet 1986; 11: 76–86
Hyatt JM, McKinnon PS, Zimmer GS, et al. The importance of pharmacokinetic/pharmacodynamic surrogate markers to outcome: focus on antibacterial agents. Clin Pharmacokinet 1995; 28: 143–60
Jacobs MR, Felmingham D, Appelbaum PC, et al. The Alexander project 1998–2000: susceptibility of pathogens isolated from community-acquired respiratory tract infection to commonly used antimicrobial agents. J Antimicrob Chemother 2003; 52: 229–46
Pallares R, Fenoli A, Linares J, et al. The epidemiology of antibiotic resistance in Streptococcus pneumoniae and the clinical relevance of resistance to cephalosporins, macrolides and quinolones. Int J Antimicrob Agents 2003; 22: S15–24
Acknowledgements
This study was financially supported by UPRES EA 3784 (Marseille) France. The authors have no conflict of interest that is directly relevant to the content of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Simon, N., Dussol, B., Sampol, E. et al. Population Pharmacokinetics of Ceftriaxone and Pharmacodynamic Considerations in Haemodialysed Patients. Clin Pharmacokinet 45, 493–501 (2006). https://doi.org/10.2165/00003088-200645050-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-200645050-00004