Abstract
Objective
To assess the pharmacokinetic and pharmacodynamic behaviour of moxifloxacin in 15 consecutive elderly patients with acute exacerbation of chronic bronchitis (AECB) treated with the fixed oral moxifloxacin 400 mg/day regimen with the intent of verifying which degree of exposure may be ensured by this standard regimen against AECB pathogens.
Methods
This was an open-label, observational, pharmacokinetic-pharmacodynamic study. Blood samples were collected at steady state at appropriate intervals. Moxifloxacin plasma concentrations were analysed by means of high-performance liquid chromatography. Standard pharmacokinetic parameters and pharmacodynamic determinants (peak concentration [Cmax]/minimum inhibitory concentration [MIC], area under the plasma concentration-time curve during the 24-hour observational period [AUC24]/MIC, pharmacodynamic breakpoints [PDBPs]) were assessed.
Results
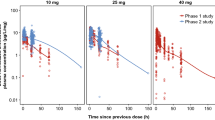
The mean estimated pharmacokinetic parameters (Cmax 4.40 mg/L at 1.4 hours, AUC24 42.67 mg ′ h/L, elimination half-life 12.55 hours, total body clearance 0.16 L/h/kg) were generally similar to those observed in both young and elderly historic controls (except for higher-dose normalised Cmax and lower volume of distribution of the central compartment). Median Cmax/MIC and AUC24/MIC ratios for moxifloxacin in the fully assessable cases were, respectively, 67.5 and 823.9 against Streptococcus pneumoniae, 25 and 310.2 against Moraxella catharralis and 416.5 and 3647.5 against Haemophilus influenzae. Mean estimates of PDBP for achieving Cmax/MIC values of 12.2 and AUC24/MIC values of 125 were 0.36 and 0.35 mg/L, respectively.
Conclusion
In patients with AECB the pharmacokinetic behaviour of moxifloxacin is not significantly altered by aging processes. This is consistent with moxifloxacin being metabolised mainly by means of phase II hepatic reactions, the activity of which was shown not to decline with age. Both the pharmacokinetic and pharmacodynamic analyses suggest that moxifloxacin 400 mg/day may be a valid therapeutic approach in the treatment of AECB in the elderly. Of note, the unmodified pharmacokinetic behaviour with no need for age-related dosage adjustments combined with the once-daily administration favouring compliance and the low potential for drug-drug pharmacokinetic interactions in case of polytherapy, make moxifloxacin particularly attractive in the treatment of elderly subpopulations at a very high risk of AECB.







Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Sethi S. Infectious etiology of acute exacerbations of chronic bronchitis. Chest 2000; 117: 380–5S
Snow V, Lascher S, Mottur-Pilson C. Evidence base for management of acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 2001; 134: 595–9
Dever LL, Shashikumar K, Johanson Jr WG. Antibiotics in the treatment of acute exacerbations of chronic bronchitis. Expert Opin Investig Drugs 2002; 11: 911–25
Niederman MS. Who should receive antibiotics for exacerbations of chronic bronchitis? A plea for more outcome-based studies. Clin Infect Dis 2004; 39: 987–9
Adams SG, Anzueto A. Antibiotic therapy in acute exacerbations of chronic bronchitis. Semin Respir Infect 2000; 15: 234–47
Pauwels R. Global initiative for chronic obstructive lung diseases (GOLD): time to act. Eur Respir J 2001; 18: 901–2
Saint S, Bent S, Vittinghoff E, et al. Antibiotics in chronic obstructive pulmonary disease exacerbations: a meta-analysis. JAMA 1995; 273: 957–60
Sethi S. The role of antibiotics in acute exacerbations of chronic obstructive pulmonary disease. Curr Infect Dis Rep 2003; 5: 9–15
Grossman RF. Management of acute exacerbation of chronic bronchitis. Can Respir J 1999; 6 Suppl. A: 40–5A
Zhanel GG, Ennis K, Vercaigne L, et al. A critical review of the fluoroquinolones: focus on respiratory infections. Drugs 2002; 62: 13–59
Soman A, Honeybourne D, Andrews J, et al. Concentrations of moxifloxacin in serum and pulmonary compartments following a single 400mg oral dose in patients undergoing fibre-optic bronchoscopy. J Antimicrob Chemother 1999; 44: 835–8
Simon N, Sampol E, Albanese J, et al. Population pharmacokinetics of moxifloxacin in plasma and bronchial secretions in patients with severe bronchopneumonia. Clin Pharmacol Ther 2003; 74: 353–63
Capitano B, Mattoes HM, Shore E, et al. Steady-state intrapulmonary concentrations of moxifloxacin, levofloxacin, and azithromycin in older adults. Chest 2004; 125: 965–73
Wilson R, Allegra L, Huchon G, et al. Short-term and long-term outcomes of moxifloxacin compared to standard antibiotic treatment in acute exacerbations of chronic bronchitis. Chest 2004; 125: 953–64
Starakis I, Gogos CA, Bassaris H. Five-day moxifloxacin therapy compared with 7-day co-amoxiclav therapy for the treatment of acute exacerbation of chronic bronchitis. Int J Antimicrob Agents 2004; 23: 129–37
Grassi C, Casali L, Curti E, et al. Efficacy and safety of short course (5-day) moxifloxacin vs 7-day ceftriaxone in the treatment of acute exacerbations of chronic bronchitis (AECB). J Chemother 2002; 14: 597–608
Ferrara AM, Fietta AM. New developments in antibacterial choice for lower respiratory tract infections in elderly patients. Drugs Aging 2004; 21: 167–86
Turnheim K. When drug therapy gets old: pharmacokinetics and pharmacodynamics in the elderly. Exp Gerontol 2003; 38: 843–53
Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. 15th Information Supplement M100-S15. Wayne (PA): Clinical and Laboratory Standards Institute, 2005
Stass H, Bottcher MF, Ochmann K. Evaluation of the influence of antacids and H2 antagonists on the absorption of moxifloxacin after oral administration of a 400mg dose to healthy volunteers. Clin Pharmacokinet 2001; 40 Suppl. 1: 39–48
Stass H, Schuhly U, Moller JG, et al. Effects of sucralfate on the oral bioavailability of moxifloxacin, a novel 8-methoxyfluoroquinolone, in healthy volunteers. Clin Pharmacokinet 2001; 40 Suppl. 1: 49–55
Stass H, Kubitza D. Effects of iron supplements on the oral bioavailability of moxifloxacin, a novel 8-methoxyfluoroquinolone, in humans. Clin Pharmacokinet 2001; 40 Suppl. 1: 57–62
Wong FA, Juzwin SJ, Flor SC. Rapid stereospecific high-performance liquid chromatographic determination of levofloxacin in human plasma and urine. J Pharm Biomed Anal 1997; 15: 765–71
Mack G. Improved high-performance liquid chromatographic determination of ciprofloxacin and its metabolites in human specimens. J Chromatogr 1992; 582: 263–7
Yamaoka K, Nakagawa T, Uno T. Application of Akaike’s information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J Pharmacokinet Biopharm 1978; 6: 165–75
Aminimanizani A, Beringer P, Jelliffe R. Comparative pharmacokinetics and pharmacodynamics of the newer fluoroquinolone antibacterials. Clin Pharmacokinet 2001; 40: 169–87
Pea F, Pavan F, Nascimben E, et al. Levofloxacin disposition in cerebrospinal fluid in patients with external ventriculostomy. Antimicrob Agents Chemother 2003; 47: 3104–8
Blaser J, Stone BB, Groner MC, et al. Comparative study with enoxacin and netilmicin in a pharmacodynamic model to determine importance of ratio of antibiotic peak concentration to MIC for bactericidal activity and emergence of resistance. Antimicrob Agents Chemother 1987; 31: 1054–60
Forrest A, Nix DE, Ballow CH, et al. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob Agents Chemother 1993; 37: 1073–81
Preston SL, Drusano GL, Berman AL, et al. Pharmacodynamics of levofloxacin: a new paradigm for early clinical trials. JAMA 1998; 279: 125–9
Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16: 31–41
Noble RE. Drug therapy in the elderly. Metabolism 2003; 52: 27–30
Stass H, Dalhoff A, Kubitza D, et al. Pharmacokinetics, safety, and tolerability of ascending single doses of moxifloxacin, a new 8-methoxy quinolone, administered to healthy subjects. Antimicrob Agents Chemother 1998; 42: 2060–5
Sullivan JT, Woodruff M, Lettieri J, et al. Pharmacokinetics of a once-daily oral dose of moxifloxacin (Bay 12-8039), a new enantiomerically pure 8-methoxy quinolone. Antimicrob Agents Chemother 1999; 43: 2793–7
Balfour JA, Lamb HM. Moxifloxacin: a review of its clinical potential in the management of community-acquired respiratory tract infections. Drugs 2000; 59: 115–39
Wise R, Andrews JM, Marshall G, et al. Pharmacokinetics and inflammatory-fluid penetration of moxifloxacin following oral or intravenous administration. Antimicrob Agents Chemother 1999; 43: 1508–10
Lubasch A, Keller I, Borner K, et al. Comparative pharmacokinetics of ciprofloxacin, gatifloxacin, grepafloxacin, levofloxacin, trovafloxacin, and moxifloxacin after single oral administration in healthy volunteers. Antimicrob Agents Chemother 2000; 44: 2600–3
Burkhardt O, Borner K, Stass H, et al. Single- and multiple-dose pharmacokinetics of oral moxifloxacin and clarithromycin, and concentrations in serum, saliva and faeces. Scand J Infect Dis 2002; 34: 898–903
Sullivan JT, Lettieri JT, Liu P, et al. The influence of age and gender on the pharmacokinetics of moxifloxacin. Clin Pharmacokinet 2001; 40 Suppl. 1: 11–8
Beyer R, Pestova E, Millichap JJ, et al. A convenient assay for estimating the possible involvement of efflux of fluoroquinolones by Streptococcus pneumoniae and Staphylococcus aureus: evidence for diminished moxifloxacin, sparfloxacin, and trovafloxacin efflux. Antimicrob Agents Chemother 2000; 44: 798–801
Allen GP, Kaatz GW, Rybak MJ. Activities of mutant prevention concentration-targeted moxifloxacin and levofloxacin against Streptococcus pneumoniae in an in vitro pharmacodynamic model. Antimicrob Agents Chemother 2003; 47: 2606–14
Stass H, Kubitza D. Pharmacokinetics and elimination of moxifloxacin after oral and intravenous administration in man. J Antimicrob Chemother 1999; 43 Suppl. B: 83–90
Bebia Z, Buch SC, Wilson JW, et al. Bioequivalence revisited: influence of age and sex on CYP enzymes. Clin Pharmacol Ther 2004; 76: 618–27
Kinirons MT, Crome P. Clinical pharmacokinetic considerations in the elderly: an update. Clin Pharmacokinet 1997; 33: 302–12
Lister PD, Sanders CC. Pharmacodynamics of levofloxacin and ciprofloxacin against Streptococcus pneumoniae. J Antimicrob Chemother 1999; 43: 79–86
Lister PD, Sanders CC. Pharmacodynamics of trovafloxacin, ofloxacin, and ciprofloxacin against Streptococcus pneumoniae in an in vitro pharmacokinetic model. Antimicrob Agents Chemother 1999; 43: 1118–23
Lister PD, Sanders CC. Pharmacodynamics of moxifloxacin, levofloxacin and sparfloxacin against Streptococcus pneumoniae. J Antimicrob Chemother 2001; 47: 811–8
Ambrose PG, Grasela DM, Grasela TH, et al. Pharmacodynamics of fluoroquinolones against Streptococcus pneumoniae in patients with community-acquired respiratory tract infections. Antimicrob Agents Chemother 2001; 45: 2793–7
Nightingale CH. Moxifloxacin, a new antibiotic designed to treat community-acquired respiratory tract infections: a review of microbiologic and pharmacokinetic-pharmacodynamic characteristics. Pharmacotherapy 2000; 20: 245–56
Acknowledgements
This research was carried out spontaneously thanks to departmental funds (Department of Experimental and Clinical Pathology and Medicine, University of Udine). The authors would like to thank Dr Loretta Franceschi for developing the analytical method and Mrs Eliana Di Terlizzi for her technical assistance. The authors have no conflicts of interest that are directly relevant to the content of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pea, F., Pavan, F., Lugatti, E. et al. Pharmacokinetic and Pharmacodynamic Aspects of Oral Moxifloxacin 400 mg/day in Elderly Patients with Acute Exacerbation of Chronic Bronchitis. Clin Pharmacokinet 45, 287–295 (2006). https://doi.org/10.2165/00003088-200645030-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-200645030-00004