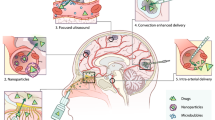
Abstract
The blood-brain barrier (BBB) is a gate that controls the influx and efflux of a wide variety of substances and consequently restricts the delivery of drugs into the central nervous system (CNS). Brain tumours may disrupt the function of this barrier locally and nonhomogeneously. Therefore, the delivery of drugs to brain tumours has long been a controversial subject. The current concept is that inadequate drug delivery is a major factor that explains the unsatisfactory response of chemosensitive brain tumours. Various strategies have been devised to circumvent the BBB in order to increase drug delivery to the CNS. The various approaches can be categorised as those that attempt to increase delivery of intravascularly administered drugs, and those that attempt to increase delivery by local drug administration. Strategies that increase delivery of intravascularly injected drugs can manipulate either the drugs or the capillary permeability of the various barriers (BBB or blood-tumour barrier), or may attempt to increase plasma concentration or the fraction of the drug reaching the tumour (high-dose chemotherapy, intra-arterial injection). Neurotoxicity is a major concern with increased penetration of drugs into the CNS or when local delivery is practised. Systemic toxicity remains the limiting factor for most methods that use intravascular delivery.
This review evaluates the strategies used to increase drug delivery in view of current knowledge of drug pharmacokinetics and its relevance to clinical studies of chemosensitive brain tumours. The main focus is on primary CNS lymphoma, as it is a chemosensitive brain tumour and its management routinely utilises specialised strategies to enhance drug delivery to the affected CNS compartments.





Similar content being viewed by others
References
Tunggal JK, Cowan DS, Shaikh H, et al. Penetration of anticancer drugs through solid tissue: a factor that limits the effectiveness of chemotherapy for solid tumors. Clin Cancer Res 1999; 5(6): 1583–6
Vick NA, Khandekar JD, Bigner DD. Chemotherapy of brain tumors: the ‘blood-brain barrier’ is not a factor. Arch Neurol 1977; 34: 523–6
Pardridge WM. CNS drug design based on principles of blood-brain barrier transport. J Neurochem 1998; 70(5): 1781–92
Rapoport SI. Modulation of blood-brain barrier permeability. J Drug Target 1996; 3: 417–25
Rapoport SI. Osmotic opening of the blood-brain barrier: principles, mechanism, and therapeutic applications. Cell Mol Neurobiol 2000; 20(2): 217–30
Tamai I, Tsuji A. Drug delivery through the blood-brain barrier. Adv Drug Deliv Rev 1996; 19: 401–24
Kroll RA, Neuwelt EA. Outwitting the blood-brain barrier for therapeutic purposes: osmotic opening and other means. Neurosurgery 1998; 42(5): 1083–100
Bartus RT. The blood-brain barrier as a target for pharmacological modulation. Curr Opin Drug Discov Devel 1999; 2(2): 152–67
Groothuis DR. The blood-brain and blood-tumor barriers: a review of strategies for increasing drug delivery. Neurooncol 2000; 2(1): 45–59
O’Neill BP, Wang CH, O’Fallon JR, et al. Primary central nervous system non-Hodgkin’s lymphoma (PCNSL): survival advantages with combined initial therapy? A final report of the North Central Cancer Treatment Group (NCCTG) Study 86-72-52. Int J Radiat Oncol Biol Phys 1999; 43(3): 559–63
Corn BW, Dolinskas C, Scott C, et al. Strong correlation between imaging response and survival among patients with primary central nervous system lymphoma: a secondary analysis of RTOG studies 83-15 and 88-06. Int J Radiat Oncol Biol Phys 2000; 47(2): 299–303
Ferreri AJ, Reni M, Villa E. Therapeutic management of primary central nervous system lymphoma: lessons from prospective trials. Ann Oncol 2000; 11(8): 927–37
Bataille B, Delwail V, Menet E, et al. Primary intracerebral malignant lymphoma: report of 248 cases. J Neurosurg 2000; 92(2): 261–6
Blay JY, Conroy T, Chevreau C, et al. High-dose methotrexate for the treatment of primary cerebral lymphomas: analysis of survival and late neurologic toxicity in a retrospective series. J Clin Oncol 1998; 16(3): 864–71
Corn BW, Marcus SM, Topham A, et al. Will primary central nervous system lymphoma be the most frequent brain tumor diagnosed in the year 2000? Cancer 1997; 79(12): 2409–13
Krogh-Jensen M, d’Amore F, Jensen MK, et al. Incidence, clinicopathological features and outcome of primary central nervous system lymphomas. Population-based data from a Danish lymphoma registry. Danish Lymphoma Study Group, LYFO. Ann Oncol 1994; 5(4): 349–54
Lutz JM, Coleman MP. Trends in primary cerebral lymphoma. Br J Cancer 1994; 70(4): 716–8
Abrey LE, Yahalom J, DeAngelis LM. Treatment for primary CNS lymphoma: the next step. J Clin Oncol 2000; 18(17): 3144–50
McAllister LD, Doolittle ND, Guastadisegni PE, et al. Cognitive outcomes and long-term follow-up results after enhanced chemotherapy delivery for primary central nervous system lymphoma. Neurosurgery 2000; 46(1): 51–61
Guha-Thakurta N, Damek D, Pollack C, et al. Intravenous methotrexate as initial treatment for primary central nervous system lymphoma: response to therapy and quality of life of patients. J Neurooncol 1999; 43(3): 259–68
Zylber-Katz E, Gomori JM, Schwartz A, et al. Pharmacokinetics of methotrexate in cerebrospinal fluid and serum after osmotic blood-brain barrier disruption in patients with brain lymphoma. Clin Pharmacol Ther 2000; 67(6): 631–41
O’Neill BP, O’Fallon JR, Earle JD, et al. Primary central nervous system non-Hodgkin’s lymphoma: survival advantages with combined initial therapy? Int J Radiat Oncol Biol Phys 1995; 33(3): 663–73
Schultz C, Scott C, Sherman W, et al. Preirradiation chemotherapy with cyclophosphamide, doxorubicin, vincristine, and dexamethasone for primary CNS lymphomas: initial report of radiation therapy oncology group protocol 88-06. J Clin Oncol 1996; 14(2): 556–64
Madara JL. Regulation of the movement of solutes across tight junctions. Annu Rev Physiol 1998; 60: 143–59
Nag S. Role of the endothelial cytoskeleton in blood-brain-barrier permeability to protein. Acta Neuropathol (Berl) 1995; 90(5): 454–60
Neuwelt EA, Frenkel EP, Rapoport S, et al. Effect of osmotic blood-brain barrier disruption on methotrexate pharmacokinetics in the dog. Neurosurgery 1980; 7(1): 36–43
Kroll RA, Pagel MA, Muldoon LL, et al. Improving drug delivery to intracerebral tumor and surrounding brain in a rodent model: a comparison of osmotic versus bradykinin modification of the blood-brain and/or blood-tumor barriers. Neurosurgery 1998; 43(4): 879–89
Dahlborg SA, Petrillo A, Crossen JR, et al. The potential for complete and durable response in nonglial primary brain tumors in children and young adults with enhanced chemotherapy delivery. Cancer J Sci Am 1998; 4(2): 110–24
Siegal T, Rubinstein R, Bokstein F, et al. In vivo assessment of the window of barrier opening after osmotic blood-brain barrier disruption in humans. J Neurosurg 2000; 92(4): 599–605
Black KL, Cloughesy T, Huang SC, et al. Intracarotid infusion of RMP-7, a bradykinin analog, and transport of gallium-68 ethylenediamine tetraacetic acid into human gliomas. J Neurosurg 1997; 86(4): 603–9
Gregor A, Lind M, Newman H, et al. Phase II studies of RMP-7 and carboplatin in the treatment of recurrent high grade glioma. RMP-7 European Study Group. J Neurooncol 1999; 44(2): 137–45
Schlageter KE, Molnar P, Lapin GD, et al. Microvessel organization and structure in experimental brain tumors: microvessel populations with distinctive structural and functional properties. Microvasc Res 1999; 58(3): 312–28
Terae S, Ogata A. Nonenhancing primary central nervous system lymphoma. Neuroradiology 1996; 38(1): 34–7
Siegal T, Rubinstein R, Tzuk-Shina T, et al. Utility of relative cerebral blood volume mapping derived from perfusion magnetic resonance imaging in the routine follow up of brain tumors. J Neurosurg 1997; 86(1): 22–7
Jain RK. Vascular and interstitial barriers to delivery of therapeutic agents in tumors. Cancer Metastasis Rev 1990; 9(3): 253–66
Huang TY, Arita N, Hayakawa T, et al. ACNU, MTX and 5-FU penetration of rat brain tissue and tumors. J Neurooncol 1999; 45(1): 9–17
Davson H. The environment of the neurons. Trends Neurosci 1978; 1: 39–41
Shibata S. Sites of origin of primary intracerebral malignant lymphoma. Neurosurgery 1989; 25(1): 14–9
Sandor V, Stark-Vancs V, Pearson D, et al. Phase II trial of chemotherapy alone for primary CNS and intraocular lymphoma. J Clin Oncol 1998; 16(9): 3000–6
Balmaceda C, Gaynor JJ, Sun M, et al. Leptomeningeal tumor in primary central nervous system lymphoma: recognition, significance, and implications. Ann Neurol 1995; 38(2): 202–9
Cher L, Glass J, Harsh GR, et al. Therapy of primary CNS lymphoma with methotrexate-based chemotherapy and deferred radiotherapy: preliminary results. Neurology 1996; 46(6): 1757–9
Siegal T. Leptomeningeal metastases: rationale for systemic chemotherapy or what is the roleofintra-CSF-chemotherapy? J Neurooncol 1998; 38(2-3): 151–7
Balis FM, Blaney SM, McCully CL, et al. Methotrexate distribution within the subarachnoid space after intraventricular and intravenous administration. Cancer Chemother Pharmacol 2000; 45(3): 259–64
Tetef ML, Margolin KA, Doroshow JH, et al. Pharmacokinetics and toxicity of high-dose intravenous methotrexate in the treatment of leptomeningeal carcinomatosis. Cancer Chemother Pharmacol 2000; 46(1): 19–26
Millot F, Rubie H, Mazingue F, et al. Cerebrospinal fluid drug levels of leukemic children receiving intravenous 5 g/m2 methotrexate. Leuk Lymphoma 1994; 14(1-2): 141–4
Chatelut E, Roche H, Plusquellec Y, et al. Pharmacokinetic modeling of plasma and cerebrospinal fluid methotrexate after high-dose intravenous infusion in children. J Pharm Sci 1991; 80(8): 730–4
Hiraga S, Arita N, Ohnishi T, et al. Rapid infusion of high-dose methotrexate resulting in enhanced penetration into cerebrospinal fluid and intensified tumor response in primary central nervous system lymphomas. J Neurosurg 1999; 91(2): 221–30
Seidel H, Andersen A, Kvaloy JT, et al. Variability in methotrexate serum and cerebrospinal fluid pharmacokinetics in children with acute lymphocytic leukemia: relation to assay methodology and physiological variables. Leuk Res 2000; 24(3): 193–9
Glantz MJ, Cole BF, Recht L, et al. High-dose intravenous methotrexate for patients with nonleukemic leptomeningeal cancer: is intrathecal chemotherapy necessary? J Clin Oncol 1998; 16(4): 1561–7
Aubree-Lecat A, Duban MC, Demignot S, et al. Influence of barrier-crossing limitations on the amount of macromolecular drug taken up by its target. J Pharmacokinet Biopharm 1993; 21(1): 75–98
Golden PL, Pollack GM. Rationale for influx enhancement versus efflux blockade to increase drug exposure to the brain. Biopharm Drug Dispos 1998; 19(4): 263–72
Wong SL, Van Belle K, Sawchuk RJ. Distributional transport kinetics of zidovudine between plasma and brain extracellular fluid/cerebrospinal fluid in the rabbit: investigation of the inhibitory effect of probenecid utilizing microdialysis. J Pharmacol Exp Ther 1993; 264(2): 899–909
Masereeuw R, Jaehde U, Langemeijer MW, et al. In vitro and in vivo transport of zidovudine (AZT) across the blood-brain barrier and the effect of transport inhibitors. Pharm Res 1994; 11(2): 324–30
Schinkel AH. P-Glycoprotein, a gatekeeper in the blood-brain barrier. Adv Drug Deliv Rev 1999; 36(2–3): 179–94
Doolittle ND, Miner ME, Hall WA, et al. Safety and efficacy of a multicenter study using intraarterial chemotherapy in conjunction with osmotic opening of the blood-brain barrier for the treatment of patients with malignant brain tumors. Cancer 2000; 88(3): 637–47
Pardridge WM. Drug delivery to the brain. J Cereb Blood Flow Metab 1997; 17(7): 713–31
Pardridge WM. Vector-mediated drug delivery to the brain. Adv Drug Deliv Rev 1999; 36(2–3): 299–321
van de Waterbeemd H, Camenisch G, Folkers G, et al. Estimation of blood-brain barrier crossing of drugs using molecular size and shape, and H-bonding descriptors. J Drug Target 1998; 6(2): 151–65
Friedman HS, Kerby T, Calvert H. Temozolomide and treatment of malignant glioma. Clin Cancer Res 2000; 6(7): 2585–97
Yoshikawa T, Sakaeda T, Sugawara T, et al. A novel chemical delivery system for brain targeting. Adv Drug Deliv Rev 1999; 36(2–3): 255–75
Groothuis DR, Benalcazar H, Allen CV, et al. Comparison of cytosine arabinoside delivery to rat brain by intravenous, intrathecal, intraventricular and intraparenchymal routes of administration. Brain Res 2000; 856(1–2): 281–90
Remsen LG, McCormick CI, Sexton G, et al. Decreased delivery and acute toxicity of cranial irradiation and chemotherapy given with osmotic blood-brain barrier disruption in a rodent model: the issue of sequence. Clin Cancer Res 1995; 1(7): 731–9
Dukic S, Heurtaux T, Kaltenbach ML, et al. Pharmacokinetics of methotrexate in the extracellular fluid of brain C6-glioma after intravenous infusion in rats. Pharm Res 1999; 16(8): 1219–25
Thyss A, Milano G, Deville A, et al. Effect of dose and repeat intravenous 24 hr infusions of methotrexate on cerebrospinal fluid availability in children with hematological malignancies. Eur J Cancer Clin Oncol 1987; 23(6): 843–7
Borsi JD, Moe PJ. A comparative study on the pharmacokinetics of methotrexate in a dose range of 0.5 g to 33.6 g/m2 in children with acute lymphoblastic leukemia. Cancer 1987; 60(1): 5–13
Neuwelt EA, Pagel M, Barnett P, et al. Pharmacology and toxicity of intracarotid adriamycin administration following osmotic blood-brain barrier modification. Cancer Res 1981; 41 (11 Pt 1): 4466–70
Dukic SF, Heurtaux T, Kaltenbach ML, et al. Influence of schedule of administration on methotrexate penetration in brain tumours. Eur J Cancer 2000; 36(12): 1578–84
Kroll RA, Pagel MA, Langone JJ, et al. Differential permeability of the blood-tumour barrier in intracerebral tumour-bearing rats: antidrug antibody to achieve systemic drug rescue. Ther Immunol 1994; 1(6): 333–41
Williams PC, Henner WD, Roman-Goldstein S, et al. Toxicity and efficacy of carboplatin and etoposide in conjunction with disruption of the blood-brain tumor barrier in the treatment of intracranial neoplasms. Neurosurgery 1995; 37(1): 17–28
Zunkeler B, Carson RE, Olson J, et al. Quantification and pharmacokinetics of blood-brain barrier disruption in humans. J Neurosurg 1996; 85(6): 1056–65
Zunkeler B, Carson RE, Olson J, et al. Hyperosmolar blood-brain barrier disruption in baboons: an in vivo study using positron emission tomography and rubidium-82. J Neurosurg 1996; 84(3): 494–502
Fortin D, McAllister LD, Nesbit G, et al. Unusual cervical spinal cord toxicity associated with intra-arterial carboplatin, intra-arterial or intravenous etoposide phosphate, and intravenous cyclophosphamide in conjunction with osmotic blood brain-barrier disruption in the vertebral artery. AJNR Am J Neuroradiol 1999; 20(10): 1794–802
Neuwelt EA, Brummett RE, Doolittle ND, et al. First evidence of otoprotection against carboplatin-induced hearing loss with a two-compartment system in patients with central nervous system malignancy using sodium thiosulfate. J Pharmacol Exp Ther 1998; 286(1): 77–84
Neuwelt EA, Brummett RE, Remsen LG, et al. In vitro and animal studies of sodium thiosulfate as a potential chemo-protectant against carboplatin-induced ototoxicity. Cancer Res 1996; 56(4): 706–9
Neuwelt EA, Barnett PA, McCormick CI, et al. Differential permeability of a human brain tumor xenograft in the nude rat: impact of tumor size and method of administration on optimizing delivery of biologically diverse agents. Clin Cancer Res 1998; 4(6): 1549–55
Robinson PJ, Rapoport SI. Model for drug uptake by brain tumors: effects of osmotic treatment and of diffusion in brain. J Cereb Blood Flow Metab 1990; 10(2): 153–61
Markowsky SJ, Zimmerman CL, Tholl D, et al. Methotrexate disposition following disruption of the blood-brain barrier. Ther Drug Monit 1991; 13(1): 24–31
Friden PM, Olson TS, Obar R, et al. Characterization, receptor mapping and blood-brain barrier transcytosis of antibodies to the human transferrin receptor. J Pharmacol Exp Ther 1996; 278(3): 1491–8
Inamura T, Black KL. Bradykinin selectively opens blood-tumor barrier in experimental brain tumors. J Cereb Blood Flow Metab 1994; 14(5): 862–70
Bartus RT, Snodgrass P, Marsh J, et al. Intravenous cereport (RMP-7) modifies topographic uptake profile of carboplatin within rat glioma and brain surrounding tumor, elevates platinum levels, and enhances survival. J Pharmacol Exp Ther 2000; 293(3): 903–11
Barth RF, Yang W, Bartus RT, et al. Enhanced delivery of boronophenylalanine for neutron capture therapy of brain tumors using the bradykinin analog Cereport (Receptor-Mediated Permeabilizer-7). Neurosurgery 1999; 44(2): 351–60
Shapiro WR, Young DF, Mehta BM. Methotrexate: distribution in cerebrospinal fluid after intravenous, ventricular and lumbar injections. N Engl J Med 1975; 293(4): 161–6
Blasberg RG, Patlak CS, Shapiro WR. Distribution of methotrexate in the cerebrospinal fluid and brain after intraventricular administration. Cancer Treat Rep 1977; 61(4): 633–41
Iacoangeli M, Roselli R, Pagano L, et al. Intrathecal chemotherapy for treatment of overt meningeal leukemia: comparison between intraventricular and traditional intralumbar route. Ann Oncol 1995; 6(4): 377–82
Bleyer WA, Poplack DG. Intraventricular versus intralumbar methotrexate for central-nervous-system leukemia: prolonged remission with the Ommaya reservoir. Med Pediatr Oncol 1979; 6(3): 207–13
Miller KT, Wilkinson DS. Pharmacokinetics of methotrexate in the cerebrospinal fluid after intracerebroventricular administration in patients with meningeal carcinomatosis and altered cerebrospinal fluid flow dynamics. Ther Drug Monit 1989; 11(3): 231–7
Chamberlain MC, Kormanik PA. Prognostic significance of 111 indium-DTPA CSF flow studies in leptomeningeal metastases. Neurology 1996; 46(6): 1674–7
Balis FM, Savitch JL, Bleyer WA, et al. Remission induction of meningeal leukemia with high-dose intravenous methotrexate. J Clin Oncol 1985; 3(4): 485–9
Morse M, Savitch J, Balis F, et al. Altered central nervous system pharmacology of methotrexate in childhood leukemia: another sign of meningeal relapse. J Clin Oncol 1985; 3(1): 19–24
Gomori JM, Heching N, Siegal T. Leptomeningeal metastases: evaluation by gadolinium enhanced spinal magnetic resonance imaging. J Neurooncol 1998; 36(1): 55–60
Freilich RJ, Krol G, DeAngelis LM. Neuroimaging and cerebrospinal fluid cytology in the diagnosis of leptomeningeal metastasis. Ann Neurol 1995; 38(1): 51–7
Siegal T, Sandbank U, Gabizon A, et al. Alteration of blood-brain-CSF barrier in experimental meningeal carcinomatosis. A morphologic and adriamycin-penetration study. J Neurooncol 1987; 4(3): 233–42
Groothuis DR, Ward S, Itskovich AC, et al. Comparison of 14C-sucrose delivery to the brain by intravenous, intraventricular, and convection-enhanced intracerebral infusion. J Neurosurg 1999; 90(2): 321–31
Laske DW, Morrison PF, Lieberman DM, et al. Chronic interstitial infusion of protein to primate brain: determination of drug distribution and clearance with single-photon emission computerized tomography imaging. J Neurosurg 1997; 87(4): 586–94
Laske DW, Youle RJ, Oldfield EH. Tumor regression with regional distribution of the targeted toxin TF-CRM107 in patients with malignant brain tumors. Nat Med 1997; 3(12): 1362–8
Kroll RA, Pagel MA, Muldoon LL, et al. Increasing volume of distribution to the brain with interstitial infusion: dose, rather than convection, might be the most important factor. Neurosurgery 1996; 38(4): 746–54
Morrison PF, Chen MY, Chadwick RS, et al. Focal delivery during direct infusion to brain: role of flow rate, catheter diameter, and tissue mechanics. Am J Physiol 1999; 277 (4 Pt 2): R1218–29
Chen MY, Lonser RR, Morrison PF, et al. Variables affecting convection-enhanced delivery to the striatum: a systematic examination of rate of infusion, cannula size, infusate concentration, and tissue-cannula sealing time. J Neurosurg 1999; 90(2): 315–20
Acknowledgements
There was no funding for the purpose of this review and there is no potential conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Siegal, T., Zylber-Katz, E. Strategies for Increasing Drug Delivery to the Brain. Clin Pharmacokinet 41, 171–186 (2002). https://doi.org/10.2165/00003088-200241030-00002
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-200241030-00002