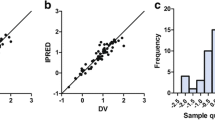
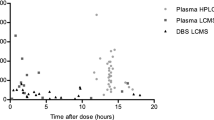
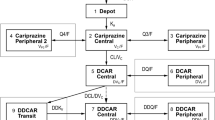
Abstract
Objective
To clarify the observed variability of haloperidol disposition in patients with psychiatric disorders.
Design
Retrospective population pharmacokinetic study.
Participants
218 Japanese patients aged 16 to 82 years who provided 391 serum haloperidol concentrations.
Methods
Routine clinical pharmacokinetic data gathered from patients receiving haloperidol were analysed to estimate population pharmacokinetic parameters with the nonlinear mixed effects model (NONMEM) computer program.
Results
The final pharmacokinetic model was CL = 42.4 • (TBW/60)0.655 • 0.814AGE≥55 • (DOSE/200)0-236 • 1.32ANTIEP and Vd = 34.4 • TBW • 0.336 AGE≥65, where CL is total body clearance (L/h), Vd is apparent volume of distribution (L), TBW is total bodyweight (kg), DOSE is daily dosage (μg/kg/day), ANTIEP = 1 for concomitant administration of antiepileptic drugs (phenobarbital, phenytoin or carbamazepine) and 0 otherwise, AGE≥55 = 1 for patient aged 55 years or over and 0 otherwise, and AGE≥65 = 1 for patient aged 65 years or over and 0 otherwise. Concomitant administration of haloperidol and antiepileptic drugs resulted in a 32% increase in haloperidol clearance. Patients aged 55 years or over showed an 18.6% reduction in clearance, and elderly patients aged 65 years or over showed a 66.4% reduction in apparent volume of distribution. Inclusion of terms for the concomitant administration of haloperidol and anti-parkinsonian drugs (amantadine, bromocriptine, biperiden, trihexyphenidyl or mazaticol) or cytochrome P450 (CYP) 2D6 substrates (levomepromazine, perphenazine, thioridazine, amitriptyline or clomipramine) did not significantly improve the estimate of haloperidol clearance.
Conclusion
Application of the findings in this study to patient care may permit selection of an appropriate initial maintenance dosage to achieve target haloperidol serum concentrations, thus enabling the clinician to achieve the desired therapeutic effect.





Similar content being viewed by others
References
Magliozzi JR, Hollister LE, Arnold KV, et al. Relationship of serum haloperidol levels to clinical response in schizophrenic patients. Am J Psychiatry 1981; 138: 365–7
Forsman A, Folsch G, Larsson M, et al. On the metabolism of haloperidol in man. Curr Ther Res 1977; 21: 606–17
Tsang MW, Shader RI, Greenblatt DJ. Metabolism of haloperidol: clinical implications and unanswered questions. J Clin Psychopharmacol 1994; 14: 159–62
Soudijn W, Wijngaarden I, Allewijn F. Distribution, excretion and metabolism of neuroleptics of the butyrophenone type. Eur J Pharmacol 1967; 1: 47–57
Gorrod JW, Fang J. On the metabolism of haloperidol. Xenobiotica 1993; 23: 495–508
Volavka J, Cooper TB. Review of haloperidol blood levels and clinical response: looking through the window. J Clin Psychopharmacol 1987; 7: 25–30
De Oliveira IR, de Sena EP, Pereira ELA, et al. Haloperidol blood levels and clinical outcome: a meta-analysis of studies relevant to testing the therapeutic window hypothesis. J Clin Pharm Ther 1996; 21: 229–36
Ulrich S, Wurthmann C, Brosz M, et al. The relationship between serum concentration and therapeutic effect of haloperidol in patients with acute schizophrenia. Clin Pharmacokinet 1998; 34: 227–63
Froemming JS, Lam YWF, Jann MW, et al. Pharmacokinetics of haloperidol. Clin Pharmacokinet 1989; 17: 396–423
ZumBrunnen TL, Jann MW. Drug interactions with antipsychotic agents: incidence and therapeutic implications. CNS Drugs 1998; 9: 381–401
Beal SL, Sheiner LB, editors. NONMEM user’s guide. San Francisco (CA): NONMEM Project Group, University of California at San Francisco, 1992
Kamisada M, Oguchi E, Yagi G. Basic evaluation of enzyme immunoassay for haloperidol level and its clinical application. J Clin Ther Med 1988; 4: 639–45
Sheiner LB, Beal SL. Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm 1981; 9: 503–12
Holley FO, Magliozzi JR, Stanski DR, et al. Haloperidol kinetics after oral and intravenous doses. Clin Pharmacol Ther 1983; 33: 477–84
Cheng YF, Paalzow LK, Bondesson U, et al. Pharmacokinetics of haloperidol in psychotic patients. Psychopharmacology 1987; 91: 410–4
Midha KK, Chakraborty BS, Ganes DA, et al. Intersubject variation in the pharmacokinetics of haloperidol and reduced haloperidol. Psychopharmacology 1989; 9: 98–104
Fang J, Baker GB, Silverstone PH, et al. Involvement of CYP3A4 and CYP2D6 in the metabolism of haloperidol. Cell Mol Neurobiol 1996; 17: 227–33
Pan LP, Wijnant P, De Vriendt C, et al. Characterization of the cytochrome P-450 isoenzymes involved in the in vitro N-dealkylation of haloperidol. Br J Clin Pharmacol 1997; 44: 557–64
Spina E, Pisani F, Perucca E. Clinically significant pharmacokinetic drug interactions with carbamazepine: an update. Clin Pharmacokinet 1996; 31: 198–214
Jann MW, Ereshefsky L, Saklad SR, et al. Effects of carbamazepine on plasma haloperidol levels. J Clin Psychopharmacol 1985; 5: 106–9
Arana GW, Goff DC, Friedman H, et al. Does carbamazepine induced reduction of plasma haloperidol levels worsen psychotic symptoms? Am J Psychiatry 1986; 143: 650–1
Kidron R, Averbuch I, Klein E, et al. Carbamazepine induced reduction in blood levels of haloperidol in chronic schizophrenia. Biol Psychiatry 1985; 20: 199–228
Hirokane G, Someya T, Takahashi S, et al. Interindividual variation of plasma haloperidol concentrations and the impact of concomitant medications: the analysis if therapeutic drug monitoring data. Ther Drug Monit 1999; 21: 82–6
Linnoila M, Viukari M, Vaisanen K, et al. Effect of anticonvulsants on plasma haloperidol and thioridazine levels. Am J Psychiatry 1980; 137: 819–21
Anderson GA. A mechanistic approach to antiepileptic drug interactions. Ann Pharmacother 1998; 32: 554–63
Acknowledgements
This study was supported by a Grant-in-Aid from the Nakatomi Foundation (E.Y.) and a Grant-in-Aid from the Japan Research Foundation for Clinical Pharmacology (E.Y.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yukawa, E., Hokazono, T., Yukawa, M. et al. Population Pharmacokinetics of Haloperidol Using Routine Clinical Pharmacokinetic Data in Japanese Patients. Clin Pharmacokinet 41, 153–159 (2002). https://doi.org/10.2165/00003088-200241020-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-200241020-00006