Abstract
This review describes the pharmacokinetics of the major drugs used for the treatment of inflammatory bowel disease. This information can be helpful for the selection of a particular agent and offers guidance for effective and well tolerated regimens.
The corticosteroids have a short elimination half-life (t1/2 β) of 1.5 to 4 hours, but their biological half-lives are much longer (12 to 36 hours). Most are moderate or high clearance drugs that are hepatically eliminated, primarily by cytochrome P450 (CYP) 3A4-mediated metabolism. Prednisone and budesonide undergo presystemic elimination. Any disease state or comedication affecting CYP3A4 activity should be taken into account when prescribing corticosteroids.
Depending on the preparation used, 10 to 50% of an oral or rectal dose of mesalazine is absorbed. Rapid acetylation in the intestinal wall and liver (t1/2 β 0.5 to 2 hours) and transport probably by P-glycoprotein affect mucosal concentrations of mesalazine, which apparently determine clinical response. Any clinical condition influencing the release and topical availability of mesalazine might modify its therapeutic potential.
Metronidazole has high (approximately 90%) oral bioavailability, with hepatic elimination characterised by a t1/2 β of 6 to 10 hours and a total clearance of about 4 L/h/kg. Ciprofloxacin is largely excreted unchanged both renally (about 45% of dose) and extrarenally (25%), with a relatively short t1/2 β (3.5 to 7 hours). Thus, renal function affects the systemic availability of ciprofloxacin.
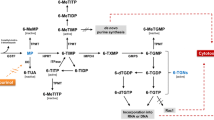
Both mercaptopurine and its prodrug azathioprine are metabolised to active compounds (6-thioguanine nucleotides; 6-TGN) by hypoxanthine-guanine phosphoribosyltransferase and to inactive metabolites by the polymorphically expressed thiopurine S-methyltransferase (TPMT) and xanthine oxidase. Patients with low TPMT activity have a higher risk of developing haemopoietic toxicity. Both mercaptopurine and azathioprine have a short t1/2 β (1 to 2 hours), but the t1/2 β of 6-TGN ranges from 3 to 13 days. Therapeutic response seems to be related to 6-TGN concentration.
Almost complete bioavailability has been observed after intramuscular and subcutaneous administration of methotrexate, which is predominantly (85%) excreted as unchanged drug with a t1/2 β of up to 50 hours. Thus, renal function is the major determinant for disposition of methotrexate. Cyclosporin is slowly and incompletely absorbed. It is extensively metabolised by CYP3A4/5 in the liver and intestine (median t1/2 β and clearance 7.9 hours and 0.46 L/h/kg, respectively), and inhibitors and inducers of CYP3A4 can modify response and toxicity.
Infliximab is predominantly distributed to the vascular compartment and eliminated with a t1/2 β between 10 and 14 days. No accumulation was observed when it was administered at intervals of 4 or 8 weeks. Methotrexate may reduce the clearance of infliximab from serum.






Similar content being viewed by others
References
Kirsner JB. Historical origins of medical and surgical therapy of inflammatory bowel disease. Lancet 1998; 352: 1303–5
Sands BE. Therapy of inflammatory bowel disease. Gastroenterology 2000; 118: S68–82
Katz S. Update in medical therapy in inflammatory bowel disease: a clinician’s view. Dig Dis 1999; 17: 163–71
Sandborn WJ, Faubion WA. Clinical pharmacology of inflammatory bowel disease therapies. Curr Gastroenterol Rep 2000; 2: 440–5
Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology 1998; 115: 182–205
Fiocchi C. Aetiopathogenesis of IBD: where do we stand. In: Rutgeerts P, Schölmerich J, Colombel J-F, et al. editors. Advances in inflammatory bowel diseases. Dordrecht: Kluwer Academic Publishers, 1999: 188–97
Ardizzone S, Bollani S, Manzionna G, et al. Inflammatory bowel disease approaching the 3rd millennium: pathogenesis and therapeutic implications? Eur J Gastroenterol Hepatol 1999; 11: 27–32
Present DH. How to do without steroids in inflammatory bowel disease. Inflamm Bowel Dis 2000; 6: 48–57
Clemett D, Markham A. Prolonged-release mesalazine. A review of its therapeutic potential in ulcerative colitis and Crohn’s disease. Drugs 2000; 59: 929–56
D’Haens GR, Rutgeerts PJ. How should corticosteroids be used in inflammatory bowel disease. Clin Immunother 1996; 5: 334–40
Thiesen A, Thomson ABR. Review article: older systemic and newer topical glucocorticosteroids and the gastrointestinal tract. Aliment Pharmacol Ther 1996; 10: 487–96
Nikolaus S, Fölsch UR, Schreiber S. Immunopharmacology of 5-aminosalicylic acid and of glucocorticoids in the therapy of inflammatory bowel disease. Hepatogastroenterology 2000; 47: 71–82
Brogden RN, McTavish D. Budesonide. An updated review of its pharmacological properties and therapeutic efficacy in asthma and rhinitis. Drugs 1992; 44: 375–407
Spencer CM, McTavish D. Budesonide. A review of its pharmacological properties and therapeutic efficacy in inflammatory bowel disease. Drugs 1995; 50: 854–72
Ryrfeldt Å, Edsbäcker S, Pauwels R. Kinetics of the epimeric glucocorticoid budesonide. Clin Pharmacol Ther 1984; 35: 525–30
Jönsson G, Åström A, Andersson P. Budesonide is metabolised by cytochrome P4503A (CYP3A) enzymes in human liver. Drug Metabol Dispos 1995; 23: 137–42
Hamedani R, Feldman RD, Feagan GB. Review article: Drug development in inflammatory bowel disease: budesonide - a model of targeted therapy. Aliment Pharmacol Ther 1997; 11 Suppl. 3: 98–108
Seidegård J. Reduction of the inhibitory effect of ketoconazole on budesonide pharmacokinetics by separation of their time of administration. Clin Pharmacol Ther 2000; 67: 13–7
Edsbäcker S, Wollmer P, Nilsson M. Pharmacokinetics and gastrointestinal transit of budesonide controlled ileal release (CIR) capsules [abstract]. Gastroenterology 1993; 104(4 Suppl.): A695
Möllmann HW, Hochhaus G, Fromm A, et al. Pharmacokinetics and pharmacodynamics of budesonide pH-modified release capsules. In: Möllemann HW, May B, editors. Glucocorticoid therapy in chronic inflammatory bowel disease — from basic science to rational therapy. Dordrecht: Kluwer Academic Publishers, 1996: 107–20
Danielson A, Edsbäcker S, Löfberg R, et al. Pharmacokinetics of budesonide enema in patients with distal ulcerative colitis or proctitis. Aliment Pharmacol Ther 1993; 7: 401–7
Möllmann HW. Pharmacological basis for the use of non-systemic oral and rectal steroids in inflammatory bowel disease. In: Tytgat GNJ, Bartelsmann JFWM, van Deeventer SJH, editors. Inflammatory bowel diseases. Dordrecht: Kluwer Academic Publishers, 1995: 633–46
Frey BM, Frey FJ. Clinical pharmacokinetics of prednisone and prednisolone. Clin Pharmacokinet 1990; 19: 126–46
Pickup ME. Clinical pharmacokinetics of prednisone and prednisolone. Clin Pharmacokinet 1979; 4: 111–28
Bergrem H. Grøttum P, Rugstad HE. Pharmacokinetics and protein binding of prednisolone after oral and intravenous administration. Eur J Clin Pharmacol 1983; 24: 415–9
Tanner A, Bochner F, Caffin J, et al. Dose-dependent prednisolone kinetics. Clin Pharmacol Ther 1979; 25: 571–8
Barth J, Damoiseaux M, Möllmann H, et al. Pharmacokinetics and pharmacodynamics of prednisolone after intravenous and oral administration. Int J Clin Pharmacol Ther Toxicol 1992; 30: 317–24
Rose JQ, Yurchak AM, Jusko WJ. Dose-dependent pharmacokinetics of prednisone and prednisolone in man. J Pharmakokinet Biopharm 1981; 9: 389–417
Pickup ME, Lowe JR, Leatham RA, et al. Dose dependent pharmacokinetics of prednisolone. Eur J Clin Pharmacol 1977; 12: 213–9
Legler UF. The pharmacokinetics of glucocorticoids. ISI Atlas Science: Pharmacology 1988; 345-50
Wald JA, Law RM, Ludwig EA, et al. Evaluation of dose-related pharmacokinetics and pharmacodynamics of prednisolone in man. J Pharmacokinet Biopharm 1992; 20: 567–89
Meffin PJ, Brooks PM, Sallustio BC. Alterations in prednisolone disposition as a result of time of administration, gender and dose. Br J Clin Pharmacol 1984; 17: 395–404
Davis M, Williams R, Chakraborty J, et al. Prednisone or prednisolone for the treatment of chronic active hepatitis? A comparison of plasma availability. Br J Clin Pharmacol 1978; 5: 501–5
Kovacs SJ, Martin DE, Everitt DE, et al. Urinary excretion of 6β-hydroxycortisol as an in vivo marker for CYP3A induction; applications and recommendations. Clin Pharmacol Ther 1998; 63: 617–22
Olivesi A. Modified elimination of prednisolone in epileptic patients on carbamazepine monotherapy, and in women using low dose oral contraceptives. Biomed Pharmacother 1986; 40: 301–8
Bartoszek M, Brenner AM, Szefcler SJ. Prednisolone and methylprednisolone kinetics in children receiving anticonvulsant therapy. Clin Pharmacol Ther 1987; 42: 424–32
Legler UF. Enhanced prednisolone elimination: a possible cause for failure of glucocorticoid therapy in Graves’ ophthalmopathy. Horm Metab Res 1987; 19: 168–70
Legler UF. Impairment of prednisolone disposition in patients with Graves’ disease taking methimazole. J Clin Endocrinol Metab 1988; 66: 221–3
Meffin PJ, Wing LMH, Sallustio BC, et al. Alterations in prednisolone disposition as a result of oral contraceptive use and dose. Br J Clin Pharmacol 1984; 17: 655–64
Legler UF, Benet LZ. Marked alterations in dose-dependent prednisolone kinetics in women taking oral contraceptives. Clin Pharmacol Ther 1986; 39: 425–9
Derendorf H. Möllmann H, Rohdewald P, et al. Kinetics of methylprednisolone and its hemisuccinate ester. Clin Pharmacol Ther 1985; 37: 502–7
Möllmann H. Rohdewald P, Barth J, et al. Comparative pharmacokinetics of methylprednisolone phosphate and hemisuccinate in high doses. Pharm Res 1988; 5: 509–13
Möllmann H, Rohdewald P, Barth J, et al. Pharmacokinetics and dose linearity testing of methylprednisolone phosphate. Biopharm Drug Dispos 1989; 10: 453–64
Al-Habet SMH, Rogers HJ. Methylprednisolone pharmacokinetics after intravenous and oral administration. Br J Clin Pharmacol 1989; 27: 285–90
Szefler SJ, Ebling WF, Georgitis JW, et al. Methylprednisolone versus prednisolone pharmacokinetics in relation to dose in adults. Eur J Clin Pharmacol 1986; 30: 323–9
Assael BM, Banfi AC, Edefonti A, et al. Disposition of pulse dose methylprednisolone in adult pediatric patients with the nephrotic syndrome. Eur J Clin Pharmacol 1982; 23: 429–33
Ludwig EA, Kong A-N, Camara DS, et al. Pharmacokinetics of methylprednisolone hemisuccinate and methylprednisolone in chronic liver disease. J Clin Pharmacol 1993; 33: 805–10
Szefler SJ, Brenner M, Jusko WJ, et al. Dose- and time-related effect of troleandomycin on methylprednisolone elimination. Clin Pharmacol Ther 1982; 32: 166–71
Glynn AM, Slaughter RL, Brass C, et al. Effects of ketoconazole on methylprednisolone pharmacokinetics and cortisol secretion. Clin Pharmacol Ther 1986; 39: 654–9
Kandrotas RJ, Slaughter RL, Brass C, et al. Ketoconazole effects on methylprednisolone disposition and their joint suppression of endogenous cortisol. Clin Pharmacol Ther 1987; 42: 470–564
Varis T, Kaukonen K-M, Kivistö J, et al. Plasma concentrations and effects of oral methylprednisolone are considerably increased by itraconazole. Clin Pharmacol Ther 1998; 64: 363–8
Varis T, Kivistö KT, Backman JT, et al. Itraconazole decreases the clearance and enhances the effects of intravenously administered methylprednisolone in healthy volunteers. Pharmacol Toxicol 1999; 85: 29–32
Varis T, Kivistö KT, Neuvonen PJ. Grapefruit juice can increase the plasma concentrations of oral methylprednisolone. Eur J Clin Pharmacol 2000; 56: 489–93
Slater KL, Ludwig EA, Lew KH, et al. Oral contraceptive effects on methylprednisolone pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther 1996; 59: 312–21
Toothaker RD, Welling G. Effect of dose size on the pharmacokinetics of intravenous hydrocortisone during endogenous hydrocortisone suppression. J Pharmacokinet Biopharm 1982; 10: 147–56
Derendorf H, Möllmann H, Barth J, et al. Pharmacokinetics and oral bioavailability of hydrocortisone. J Clin Pharmacol 1991; 31: 473–6
Möllmann H, Barth J, Möllmann C, et al. Pharmacokinetics and rectal bioavailability of hydrocortisone acetate. J Pharm Sci 1991; 80: 835–6
Svartz N. Salazopyrin, a new sulfanilamide preparation: A. Therapeutic results in rheumatic polyarthritis. B. Therapeutic results in ulcerative colitis. C. Toxic manifestations in treatment with sulfanilamide preparations. Acta Med Scand 1942; 110: 557–90
Azad Khan AK, Piris J, Truelove SC. An experiment to determine the active therapeutic moiety of sulphasalazine. Lancet 1977; 2: 892–5
van Hees PA, Bakker JH, van Tongeren JH. Effect of sulphapyridine, 5-aminosalicylic acid, and placebo in patients with idiopathic proctitis: a study to determine the active therapeutic moiety of sulphasalazine. Gut 1980; 21: 632–5
Klotz U, Maier K, Fischer C, et al. Therapeutic efficacy of sulfasalazine and its metabolites in patients with ulcerative colitis and Crohn’s disease. N Engl J Med 1980; 303: 1499–502
Järnerot G. Newer 5-aminosalicylic acid based drugs in chronic inflammatory bowel disease. Drugs 1989; 37: 73–86
Brogden RN, Sorkin EM. Mesalazine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in chronic inflammatory bowel disease. Drugs 1989; 38: 500–23
Thomson AB. Review article: new developments in the use of 5-aminosalicylic acid in patients with inflammatory bowel disease. Aliment Pharmacol Ther 1991; 5: 449–70
Prakash A, Markham A. Oral delayed-release mesalazine: a review of its use in ulcerative colitis and Crohn’s disease. Drugs 1999; 57: 383–408
Tromm A, Griga T, May B. Oral mesalazine for the treatment of Crohn’s disease: clinical efficacy with respect to pharmacokinetic properties. Hepatogastroenterology 1999; 46: 3124–35
Klotz U. Clinical pharmacokinetics of sulphasalazine, its metabolites and other prodrugs of 5-aminosalicylic acid. Clin Pharmacokinet 1985; 10: 285–302
Klotz U, Maier KE. Pharmacology and pharmacokinetics of 5-aminosalicylic acid. Dig Dis Sci 1987; 32: 46S–50S
Klotz U. Pharmacokinetic properties of various preparations of 5-aminosalicylic acid (5-ASA) and budesonide. Med Klin 1999; 94 Suppl. 1: 16–22
De Vos M. Clinical pharmacokinetics of slow release mesalazine. Clin Pharmacokinet 2000; 39: 85–97
Myers B, Evans DNW, Rhodes J, et al. Metabolism and urinary excretion of 5-aminosalicylic acid in healthy volunteers when given intravenously or released for absorption at different sites in the gastrointestinal tract. Gut 1987; 28: 196–200
Bondesen S, Hegnhøj J, Larsen F, et al. Pharmacokinetics of 5-aminosalicylic acid in man following administration of intravenous bolus and per os slow-release formulation. Dig Dis Sci 1991; 36: 1735–40
Allgayer H, Ahnfelt NO, Kruis W, et al. Colonic N-acetylation of 5-aminosalicylic acid in inflammatory bowel disease. Gastroenterology 1989; 97: 38–41
Meese CO, Fischer C, Klotz U. Is N-acetylation of 5-amino-salicylic acid reversible in man? Br J Clin Pharmacol 1984; 18: 612–5
Vree TB, Dammers E, Exler PS, et al. Saturable active tubular reabsorption in the renal clearance of mesalazine in human volunteers. Clin Drug Invest 2000; 20: 35–42
Klotz U. Pharmacokinetic properties of 5-aminosalicylic acid (mesalazine). In: Goebell H, Peskar BM, Malchow H, editors. Inflammatory bowel diseases — basic research and clinical implications. Dordrecht: MTS Press, 1988: 339–47
Klotz U, Harings-Kaim A. Negligible excretion of 5-aminosalicylic acid in breast milk. Lancet 1993; 342: 618–619
Goebell H, Klotz U, Nehlsen B, et al. Oroileal transit of slow release 5-aminosalicylic acid. Gut 1993; 34: 669–75
Layer PH, Goebell H, Keller J, et al. Delivery and fate of oral mesalamine microgranules within the human small intestine. Gastroenterology 1995; 108: 1427–33
Zhou SY, Fleisher D, Pao LH, et al. Intestinal metabolism and transport of 5-aminosalicylate. Drug Metab Dispos 1999; 27: 479–85
Proudfoot LE. Yazdanian. Mechanisms of transport and structure-permeability relationship of sulfasalazine and its analogs in Caco-2 cell monolayers. Pharm Res 2000; 17: 1168–74
Yacyshyn B, Maksymowych W, Bowen-Yacyshyn MB. Differences in P-glycoprotein-170 expression and activity between Crohn’s disease and ulcerative colitis. Hum Immunol 1999; 60: 677–687
Farrell RJ, Murphy A, Long A, et al. High multidrug resistance (P-glycoprotein 170) expression in inflammatory bowel disease patients who fail medical therapy. Gastroenterology 2000; 118: 279–88
Hussain FN, Ajjan RA, Riley SA. Dose loading with delayed-release mesalazine: a study of tissue drug concentrations and standard pharmacokinetic parameters. Br J Clin Pharmacol 2000; 49: 323–30
De Vos M, Verdievel H, Schoonjans R, et al. Concentrations of 5-ASA and Ac-5-ASA in human ileocolonic biopsy homogenates after oral 5-ASA preparations. Gut 1992; 33: 1338–42
Wadworth AN, Fitton A. Olsalazine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in inflammatory bowel disease. Drugs 1991; 41: 647–64
Støa-Birketvedt G, Florholmen J. The systemic load and efficient delivery of active 5-aminosalicylic acid in patients with ulcerative colitis on treatment with olsalazine or mesalazine. Aliment Pharmacol Ther 1999; 13: 357–61
Prakash A, Spencer CM. Balsalazide. Drugs 1998; 56: 83–9
Lowry PW, Szumlanski CL, Weinshilboum RM, et al. Balsalazide and azathioprine or 6-mercaptopurine: evidence for a potentially serious drug interaction. Gastroenterology 1999; 116: 1505–6
Frieri G, Pimpo MT, Andreoli A, et al. Prevention of post-operative recurrence of Crohn’s disease requires adequate mucosal concentration of mesalazine. Aliment Pharmacol Ther 1999; 13: 557–82
Frieri G, Pimpo MT, Palumbo G, et al. Anastomotic configuration and mucosal 5-aminosalicylic acid (5-ASA) concentrations in patients with Crohn’s disease: a GISC study. Am J Gastroenterol 2000; 95: 1486–90
Frieri G, Giacomelli R, Pimpo M, et al. Mucosal 5-aminosalicylic acid concentration inversely correlates with severity of colonic inflammation in patients with ulcerative colitis. Gut 2000; 47: 410–4
Gionchetti P, Campieri M, Belluzzi A, et al. Bioavailability of single and multiple doses of a new oral formulation of 5-ASA in patients with inflammatory bowel disease and healthy volunteers. Aliment Pharmacol Ther 1994; 8: 535–40
Norlander B, Gotthard R, Ström M. Steady-state pharmacokinetics of enteric coated 5-amino-salicylic acid tablets in healthy volunteers and in patients with Crohn’s disease or ulcerative colitis. Aliment Pharmacol Ther 1991; 5: 291–300
van Bodegraven AA, Boer RO, Lourens J, et al. Distribution of mesalazine enemas in active and quiescent ulcerative colitis. Aliment Pharmacol Ther 1996; 10: 327–32
Campieri M, Corbelli C, Gionchetti P, et al. Spread and distribution of 5-ASA colonic foam and 5-ASA enema in patients with ulcerative colitis. Dig Dis Sci 1992; 37: 1890–7
Schoonjans R, de Vos M, Schelfhout A-M, et al. Distribution and concentration of 5-aminosalicylic acid in rectosigmoid biopsy specimen after rectal administration. Dis Colon Rectum 1996; 39: 788–93
Almer S, Norlander B, Ström M, et al. Steady-state pharmacokinetics of a new 4-gram 5-aminosalicylic acid retention enema in patients with ulcerative colitis in remission. Scand J Gastroenterol 1991; 327-35
Jacobsen BA, Abildgaard K, Rasmussen HH, et al. Availability of mesalazine (5-aminosalicylic acid) from enemas and suppositories during steady-state conditions. Scand J Gastroenterol 1991; 26: 374–8
Williams CN, Haber G, Aquino JA. Double-blind, placebo-controlled evaluation of 5-ASA suppositories in active distal proctitis and measurement of extent of spread using 99mTc-labelled 5-ASA suppositories. Dig Dis Sci 1987; 32: 71S–5S
Wilding IR, Kewnyon CJ, Hooper G. Gastrointestinal spread of oral prolonged-release mesalazine microgranules (Pentasa) dosed as either tablets or sachet. Aliment Pharmacol Ther 2000; 14: 163–9
de Mey C, Meineke I. Prandial and diurnal effects on the absorption of orally administered enteric coated 5-aminosalicylic acid (5-ASA). Br J Clin Pharmacol 1992; 33: 179–82
Wiltink EHH, Mulder JJ, Stolk LML, et al. Absorption of oral mesalazine-containing preparations and the influence of famotidine on the absorption. Scand J Gastroenterol 1990; 25: 579–84
Rijk MCM, van Hogezard RA, van Schaik A, et al. Disposition of 5-aminosalicylic acid from 5-aminosalicylate-delivering drug during accelerated intestinal transit in healthy volunteers. Scand J Gastroenterol 1989; 24: 1179–85
Rijk MCM, van Schaik A, van Tongeren JHM. Disposition of mesalazine from mesalazine-delivering drugs in patients with inflammatory bowel disease, with and without diarrhoea. Scand J Gastroenterol 1992; 27: 863–8
Laursen LS, Stokholm M, Bukhave J, et al. Disposition of 5-aminosalicylic acid by olsalazine and three mesalazine preparations in patients with ulcerative colitis: comparison of intraluminal colonic concentrations, serum values, and urinary excretion. Gut 1990; 31: 1271–6
Fischer C, Maier K, Stumpf E, et al. Disposition of 5-amino-salicylic acid, the active metabolite of sulphasalazine, in man. Eur J Clin Pharmacol 1983; 25: 511–5
Cangemi JR. The role of antibiotics in Crohn’s disease. Dig Dis 1999; 17: 1–5
Chapman RW, Selby WS, Jewell DP. Controlled trial of intravenous metronidazole as an adjunct to corticosteroids in severe ulcerative colitis. Gut 1986; 27: 1210–2
Hanauer SB. Inflammatory bowel disease. N Engl J Med 1996; 334: 841–8
Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults. American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol 1997; 92: 204–11
Sandborn WJ, McLeod R, Jewell DP. Medical therapy for induction and maintenance of remission in pouchitis: a systematic review. Inflamm Bowel Dis 1999; 5: 33–9
Hulten K, Almashhrawi A, El-Zaatari FA, et al. Antibacterial therapy for Crohn’s disease: a review emphasizing therapy directed against mycobacteria. Dig Dis Sci 2000; 45: 445–56
Ursing B, Alm T, Barany F, et al. A comparative study of metronidazole and sulfasalazine for active Crohn’s disease: the cooperative Crohn’s disease study in Sweden. II. Result. Gastroenterology 1982; 83: 550–62
Sutherland L, Singleton J, Sessions J, et al. Double blind, placebo controlled trial of metronidazole in Crohn’s disease. Gut 1991; 32: 1071–5
Prantera C, Zannoni F, Scribano ML, et al. An antibiotic regimen for the treatment of active Crohn’s disease: a randomized, controlled clinical trial of metronidazole plus ciprofloxacin. Am J Gastroenterol 1996; 91: 328–32
Edwards DI. Mechanism of antimicrobial action of metronidazole. J Antimicrob Chemother 1979; 5: 499–502
Shaffer JL, Kershaw A, Houston JB. Disposition of metronidazole and its effects on sulphasalazine metabolism in patients with inflammatory bowel disease. Br J Clin Pharmacol 1986; 21: 431–5
Lau AH, Lam NP, Piscitelli SC, et al. Clinical pharmacokinetics of metronidazole and other nitroimidazole anti-infectives. Clin Pharmacokinet 1992; 23: 328–64
Lamp KC, Freeman CD, Klutman NE, et al. Pharmacokinetics and pharmacodynamics of the nitroimidazole antimicrobials. Clin Pharmacokinet 1999; 36: 353–73
Bergan T, Bjerke PE, Fausa O. Pharmacokinetics of metronidazole in patients with enteric disease compared to normal volunteers. Chemotherapy 1981; 27: 233–8
Loft S, Egsmose C, Sonne J, et al. Metronidazole elimination is preserved in the elderly. Hum Experiment Toxicol 1990; 9: 155–9
Roux AF, Moirot E, Delhotal B, et al. Metronidazole kinetics in patients with acute renal failure on dialysis: a cumulative study. Clin Pharmacol Ther 1984; 36: 363–8
Greenbloom SL, Steinhart AH, Greenberg GR. Combination ciprofloxacin and metronidazole for active Crohn’s disease. Can J Gastroenterol 1998; 12: 53–6
Turunen UM, Farkkila MA, Hakala K, et al. Long-term treatment of ulcerative colitis with ciprofloxacin: a prospective, double-blind, placebo-controlled study. Gastroenterology 1998; 115: 1072–8
Turunen U, Färkkilä M, Valtonen V, et al. Long-term outcome of ciprofloxacin treatment in severe perianal or fistulous Crohn’s disease [abstract]. Gastroenterology 1993; 104: A793
Bergan T, Thorsteinsson SB, Kolstad IM, et al. Pharmacokinetics of ciprofloxacin after intravenous and increasing oral doses. Eur J Clin Microbiol 1986; 5: 187–92
Plaisance KI, Drusano GL, Forrest A, et al. Effect of dose size on bioavailability of ciprofloxacin. Antimicrob Agents Chemother 1987; 31: 956–8
Drusano GL, Standiford HC, Plaisance K, et al. Absolute oral bioavailability of ciprofloxacin. Antimicrob Agents Chemother 1986; 30: 444–6
Joos B, Ledergerber B, Flepp M, et al. Comparison of high-pressure liquid chromatography and bioassay for determination of ciprofloxacin in serum and urine. Antimicrob Agents Chemother 1985; 27: 353–6
Höffken G, Lode H, Prinzing C, et al. Pharmacokinetics of ciprofloxacin after oral and parenteral administration. Antimicrob Agents Chemother 1985; 27: 375–9
Campoli-Richards DM, Monk JP, Price A, et al. Ciprofloxacin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs 1988; 35: 373–447
Ball AP, Fox C, Ball ME, et al. Pharmacokinetics of oral ciprofloxacin, 100 mg single dose, in volunteers and elderly patients. J Antimicrob Chemother 1986; 17: 629–35
Bayer A, Gajewska A, Stephens M, et al. Pharmacokinetics of ciprofloxacin in the elderly. Respiration 1987; 51: 292–5
Gasser TC, Ebert SC, Graversen PH, et al. Ciprofloxacin pharmacokinetics in patients with normal and impaired renal function. Antimicrob Agents Chemother 1987; 31: 709–12
Boelaert J, Valcke Y, Schurgers M, et al. The pharmacokinetics of ciprofloxacin in patients with impaired renal function. J Antimicrob Chemother 1985; 16: 87–93
Leiper K, Morris AI, Rhodes JM. Open label trial of oral clarithromycin in active Crohn’s disease. Aliment Pharmacol Ther 2000; 14: 801–6
Fietta A, Merlini C, Gialdroni Grassi GJ. Requirements for intracellular accumulation and release of clarithromycin and azithromycin by human phagocytes. Chemotherapy 1997; 9: 23–31
Prantera C, Kohn A, Mangiarotti R, et al. Antimycobacterial therapy in Crohn’s disease: results of a controlled, double-blind trial with a multiple antibiotic regimen. Am J Gastroenterol 1994; 89: 513–8
Hermon-Taylor J, Barnes N, Clarke C, et al. Mycobacterium paratuberculosis cervical lymphadenitis, followed five years later by terminal ileitis similar to Crohn’s disease. BMJ 1998; 316: 449–53
Kees F, Wellenhofer M, Grobecker H. Serum and cellular pharmacokinetics of clarithromycin 500 mg q.d. and 250 mg b.i.d. in volunteers. Infection 1995; 23: 168–72
Rodvold KA. Clinical pharmacokinetics of clarithromycin. Clin Pharmacokinet 1999; 37: 385–98.
Fraschini F, Scaglione F, Demartini G. Clarithromycin clinical pharmacokinetics. Clin Pharmacokinet 1993; 25: 189–204
Rodrigues AD, Roberts EM, Mulford DJ, et al. Oxidative metabolism of clarithromycin in the presence of human liver microsomes. Major role for the cytochrome P4503A (CYP3A) subfamily. Drug Metab Dispos 1997; 25: 623–30
Willoughby JM, Beckett J, Kumar PJ, et al. Controlled trial of azathioprine in Crohn’s disease. Lancet 1971; 2: 944–7
Candy S, Wright J, Gerber M, et al. A controlled double blind study of azathioprine in the management of Crohn’s disease. Gut 1995; 37: 674–8
Present DH, Korelitz BI, Wisch N, et al. Treatment of Crohn’s disease with 6-mercaptopurine. A long-term, randomized, double-blind study. N Engl J Med 1980; 302: 981–7
Pearson DC, May GR, Fick GH, et al. Azathioprine and 6-mercaptopurine in Crohn disease. A meta-analysis. Ann Intern Med 1995; 123: 132–42
Rosenberg JL, Wall AJ, Levin B, et al. A controlled trial of azathioprine in the management of chronic ulcerative colitis. Gastroenterology 1975; 69: 96–9
Hawthorne AB, Logan RF, Hawkey CJ, et al. Randomised controlled trial of azathioprine withdrawal in ulcerative colitis. BMJ 1992; 305: 20–2
Markowitz J, Rosa J, Grancher K, et al. Long-term 6-mercaptopurine treatment in adolescents with Crohn’s disease. Gastroenterology 1990; 99: 1347–51
Sandborn WJ. Azathioprine: state of the art in inflammatory bowel disease. Scand J Gastroenterol 1998; 225 Suppl.: 92–9
Chalmers AH. Studies on the mechanism of formation of 5-mercapto-1-methyl-4-nitroimidazole, a metabolite of the immunosuppressive drug azathioprine. Biochem Pharmacol 1974; 23: 1891–901
Chabner BA, Allegra CJ, Curt GA, et al. Antineoplastic agents. In: Hardman JG, Limbird LE, editors. Goodman & Gilman’s the pharmacological basis of therapeutics. 9th ed. New York: McGraw-Hill, 1996: 1233–87
Tidd DM, Paterson AR. A biochemical mechanism for the delayed cytotoxic reaction of 6-mercaptopurine. Cancer Res 1974; 34: 738–46
Lennard L, Van Loon JA, Weinshilboum RM. Pharmacogenetics of acute azathioprine toxicity: relationship to thiopurine methyltransferase genetic polymorphism. Clin Pharmacol Ther 1989; 46: 149–54
Evans WE, Horner M, Chu YQ, et al. Altered mercaptopurine metabolism, toxic effects, and dosage requirement in a thiopurine methyltransferase-deficient child with acute lymphocytic leukemia. J Pediatr 1991; 119: 985–9
Schütz E, Gummert J, Mohr F, et al. Azathioprine-induced myelosuppression in thiopurine methyltransferase deficient heart transplant recipient. Lancet 1993; 34: 436
Relling MV, Hancock ML, Rivera GK, et al. Mercaptopurine therapy intolerance and heterozygosity at the thiopurine S-methyltransferase gene locus. J Natl Cancer Inst 1999; 91: 2001–8
McLeod HL, Krynetski EY, Relling MV, et al. Genetic polymorphism of thiopurine methyltransferase and its clinical relevance for childhood acute lymphoblastic leukemia. Leukemia 2000; 14: 567–72
Van Os EC, Zins BJ, Sandborn WJ, et al. Azathioprine pharmacokinetics after intravenous, oral, delayed release oral and rectal foam administration. Gut 1996; 39: 63–8
Zins BJ, Sandborn WJ, McKinney JA, et al. A dose-ranging study of azathioprine pharmacokinetics after single-dose administration of a delayed-release oral formulation. J Clin Pharmacol 1997; 37: 38–46
Gervasio J, Tabba M, Lima J, et al. Comparison of azathioprine (AZA) and 6-mercaptopurine (6-MP) plasma concentrations after administration of AZA solution into the stomach, jejunum, or caecum [abstract]. Gastroenterology 1998; 144: A985
Present DH, Meltzer SJ, Krumholz MP, et al. 6-Mercaptopurine in the management of inflammatory bowel disease: short- and long-term toxicity. Ann Intern Med 1989; 111: 641–9
Zimm S, Collins JM, O’Neill D, et al. Inhibition of first-pass metabolism in cancer chemotherapy: interaction of 6-mercaptopurine and allopurinol. Clin Pharmacol Ther 1983; 34: 810–7
Arndt CA, Balis FM, McCully CL, et al. Bioavailability of low-dose vs high-dose 6-mercaptopurine. Clin Pharmacol Ther 1988; 43: 588–91
Cuffari C, Hunt S, Bayless TM. Enhanced bioavailability of azathioprine compared to 6-mercaptopurine therapy in inflammatory bowel disease: correlation with treatment efficacy. Aliment Pharmacol Ther 2000; 14: 1009–14
Adler DJ, Korelitz BI. The therapeutic efficacy of 6-mercaptopurine in refractory ulcerative colitis. Am J Gastroenterol 1990; 85: 717–22
Munkholm P, Langholz E, Davidsen M, et al. Frequency of glucocorticoid resistance and dependency in Crohn’s disease. Gut 1994; 35: 360–2
Sandborn WJ. A review of immune modifier therapy for inflammatory bowel disease: azathioprine, 6-mercaptopurine, cyclosporine, and methotrexate. Am J Gastroenterol 1996; 91: 423–33
Ewe K, Press AG, Singe CC, et al. Azathioprine combined with prednisolone or monotherapy with prednisolone in active Crohn’s disease. Gastroenterology 1993; 105: 367–72
Pearson DC, May GR, Fick G, et al. Azathioprine for maintaining remission of Crohn’s disease. Cochrane Database Syst Rev 2000; (2): CD000067
Lennard L, Van Loon JA, Lilleyman JS, et al. Thiopurine pharmacogenetics in leukemia: correlation of erythrocyte thiopurine methyltransferase activity and 6-thioguanine nucleotide concentrations. Clin Pharmacol Ther 1987; 41: 18–25
Lennard L, Lilleyman JS, Van Loon J, et al. Genetic variation in response to 6-mercaptopurine for childhood acute lymphoblastic leukaemia. Lancet 1990; 336: 225–9
Lennard L. The clinical pharmacology of 6-mercaptopurine. Eur J Clin Pharmacol 1992; 43: 329–39
Sandborn WJ, Van Os EC, Zins BJ, et al. An intravenous loading dose of azathioprine decreases the time to response in patients with Crohn’s disease. Gastroenterology 1995; 109: 1808–17
Lilleyman JS, Lennard L. Mercaptopurine metabolism and risk of relapse in childhood lymphoblastic leukaemia. Lancet 1994; 343: 1188–90
Cuffari C, Theoret Y, Latour S, et al. 6-Mercaptopurine metabolism in Crohn’s disease: correlation with efficacy and toxicity. Gut 1996; 39: 401–6
Dubinsky MC, Lamothe S, Yang HY, et al. Pharmacogenomics and metabolite measurement for 6-mercaptopurine therapy in inflammatory bowel disease. Gastroenterology 2000; 118: 705–13
Sandborn WJ, Tremaine WJ, Wolf DC, et al. Lack of effect of intravenous administration on time to respond to azathioprine for steroid-treated Crohn’s disease. North American Azathioprine Study Group. Gastroenterology 1999; 117: 527–35
Lysaa RA, Giverhaug T, Wold HL, et al. Inhibition of human thiopurine methyltransferase by furosemide, bendroflumethiazide and trichlormethiazide. Eur J Clin Pharmacol 1996; 49: 393–6
Klemetsdal B, Straume B, Wist E, et al. Identification of factors regulating thiopurine methyltransferase activity in a Norwegian population. Eur J Clin Pharmacol 1993; 44: 147–52
Pazmino PA, Sladek SL, Weinshilboum RM. Thiol S-methylation in uremia: erythrocyte enzyme activities and plasma inhibitors. Clin Pharmacol Ther 1980; 28: 356–67
McLeod HL, Relling MV, Liu Q, et al. Polymorphic thiopurine methyltransferase in erythrocytes is indicative of activity in leukemic blasts from children with acute lymphoblastic leukemia. Blood 1995; 85: 1897–902
Woodson LC, Ames MM, Selassie CD, et al. Thiopurine methyltransferase. Aromatic thiol substrates and inhibition by benzoic acid derivatives. Mol Pharmacol 1983; 24: 471–8
Szumlanski CL, Weinshilboum RM. Sulphasalazine inhibition of thiopurine methyltransferase: possible mechanism for interaction with 6-mercaptopurine and azathioprine. Br J Clin Pharmacol 1995; 39: 456–9
Lewis LD, Benin A, Szumlanski CL, et al. Olsalazine and 6-mercaptopurine-related bone marrow suppression: a possible drug-drug interaction. Clin Pharmacol Ther 1997; 62: 464–75
Langford CA, Klippel JH, Balow JE, et al. Use of cytotoxic agents and cyclosporine in the treatment of autoimmune disease. Part 2: Inflammatory bowel disease, systemic vasculitis, and therapeutic toxicity. Ann Intern Med 1998; 129: 49–58
Weinblatt ME, Coblyn JS, Fox DA, et al. Efficacy of low-dose methotrexate in rheumatoid arthritis. N Engl J Med 1985; 312: 818–22
Willkens RF, Williams HJ, Ward JR, et al. Randomized, double-blind, placebo controlled trial of low-dose pulse methotrexate in psoriatic arthritis. Arthritis Rheum 1984; 27: 376–81
Chu E, Allegra CJ. Antifolates. In: Chabner BA, Longo DL, editors. Cancer chemotherapy and biotherapy. Philadelphia: Lippincott-Raven, 1996: 109–48
Kozarek RA, Patterson DJ, Gelfand MD, et al. Methotrexate induces clinical and histologic remission in patients with refractory inflammatory bowel disease. Ann Intern Med 1989; 110: 353–6
Feagan BG, Rochon J, Fedorak RN, et al. Methotrexate for the treatment of Crohn’s disease. The North American Crohn’s Study Group Investigators. N Engl J Med 1995; 332: 292–7
Lemann M, Chamiot-Prieur C, Mesnard B, et al. Methotrexate for the treatment of refractory Crohn’s disease. Aliment Pharmacol Ther 1996; 10: 309–14
Feagan BG, Fedorak RN, Irvine EJ, et al. A comparison of methotrexate with placebo for the maintenance of remission in Crohn’s disease. North American Crohn’s Study Group Investigators. N Engl J Med 2000; 342: 1627–32
Oren R, Arber N, Odes S, et al. Methotrexate in chronic active ulcerative colitis: a double-blind, randomized, Israeli multicenter trial. Gastroenterology 1996; 110: 1416–21
Oren R, Moshkowitz M, Odes S, et al. Methotrexate in chronic active Crohn’s disease: a double-blind, randomized, Israeli multicenter. Am J Gastroenterol 1997; 92: 2203–9
Arora S, Katkov WN, Cooley J, et al. A double-blind, randomized, placebo-controlled trial of methotrexate in Crohn’s disease. Gastroenterology 1993; 102: A591
Moshkowitz M, Oren R, Tishler M, et al. The absorption of low-dose methotrexate in patients with inflammatory bowel disease. Aliment Pharmacol Ther 1997; 11: 569–73
Balis FM, Savitch JL, Bleyer WA. Pharmacokinetics of oral methotrexate in children. Cancer Res 1983; 43: 2342–5
Wan SH, Huffman DH, Azarnoff DL, et al. Effect of route of administration and effusions on methotrexate pharmacokinetics. Cancer Res 1974; 34: 3487–91
Cohen MH, Creaven PJ, Fossieck BE, et al. Effect of oral prophylactic broad spectrum nonabsorbable antibiotics on the gastrointestinal absorption of nutrients and methotrexate in small cell bronchogenic carcinoma patients. Cancer 1976; 38: 1556–9
Egan LJ, Sandborn WJ, Mays DC, et al. Systemic and intestinal pharmacokinetics of methotrexate in patients with inflammatory bowel disease. Clin Pharmacol Ther 1999; 65: 29–39
Seideman P, Beck O, Eksborg S, et al. The pharmacokinetics of methotrexate and its 7-hydroxy metabolite in patients with rheumatoid arthritis. Br J Clin Pharmacol 1993; 35: 409–12
Shen DD, Azarnoff DL. Clinical pharmacokinetics of methotrexate. Clin Pharmacokinet 1978; 3: 1–13
Bannwarth B, Pehourcq F, Schaeverbeke T, et al. Clinical pharmacokinetics of low-dose pulse methotrexate in rheumatoid arthritis. Clin Pharmacokinet 1996; 30: 194–210
Egan LJ, Sandborn WJ, Tremaine WJ, et al. Arandomized dose-response and pharmacokinetic study of methotrexate for refractory inflammatory Crohn’s disease and ulcerative colitis. Aliment Pharmacol Ther 1999; 13: 1597–604
Rooney TW, Furst DE, Koehnke R, et al. Aspirin is not associated with more toxicity than other nonsteroidal antiinflammatory drugs in patients with rheumatoid arthritis treated with methotrexate. J Rheumatol 1993; 20: 1297–302
Wilke WS, Mackenzie AH. Methotrexate therapy in rheumatoid arthritis. Current status. Drugs 1986; 32: 103–13
Ahern M, Booth J, Loxton A, et al. Methotrexate kinetics in rheumatoid arthritis: is there an interaction with nonsteroidal antiinflammatory drugs? J Rheumatol 1988; 15: 1356–60
Mathers D, Russel AS. Methotrexate. In: Dixon VJA, Furst DE, editors. Second line agents in the treatment of rheumatic diseases. New York: Marcel Dekker, 1992: 287–310
Bannwarth B, Labat L, Moride Y, et al. Methotrexate in rheumatoid arthritis. An update. Drugs 1994; 47: 25–50
Lafforgue P, Monjanel-Mouterde S, Is there an interaction between low doses of corticosteroids and methotrexate in patients with rheumatoid arthritis? A pharmacokinetic study in 33 patients. J Rheumatol 1993; 20: 263–7
Al-Awadhi A, Dale P, McKendry RJ. Pancytopenia associated with low dose methotrexate therapy. A regional survey. J Rheumatol 1993; 20: 1121–5
Govert JA, Patton S, Fine RL. Pancytopenia from using trimethoprim and methotrexate. Ann Intern Med 1992; 117: 877–8
Stewart CF, Evans WE. Drug-drug interactions with antirheumatic agents: review of selected clinically important interactions. J Rheumatol 1990; 22 (Suppl.): 16–23
Feutren G. The optimal use of cyclosporin A in autoimmune diseases. J Autoimmun 1992; 5 Suppl. A: 183–95
Fathman CG, Myers BD, Cyclosporine therapy for autoimmune disease. N Engl J Med 1992; 326: 1693–5
Denton MD, Magee CC, Sayegh MH. Immunosuppressive strategies in transplantation. Lancet 1999; 353: 1083–91
Hess AD, Thoburn CJ. Immunobiology and immunotherapeutic implications of syngeneic/autologous graft-versus-host disease. Immunol Rev 1997; 157: 111–23
Borel JF, Feurer C, Gubler HU, et al. Biological effects of cyclosporin A: a new antilymphocytic agent. Agents Actions 1976; 6: 468–75
Lichtiger S, Present DH, Kornbluth A, et al. Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N Engl J Med 1994; 330: 1841–5
Carbonnel F, Boruchowicz A, Duclos B, et al. Intravenous cyclosporine in attacks of ulcerative colitis: short-term and long-term responses. Dig Dis Sci 1996; 41: 2471–6
Santos J, Baudet S, Casellas F, et al. Efficacy of intravenous cyclosporine for steroid refractory attacks of ulcerative colitis. J Clin Gastroenterol 1995; 20: 285–9
Egan LJ, Sandborn WJ, Tremaine WJ. Clinical outcome following treatment of refractory inflammatory and fistulizing Crohn’s disease with intravenous cyclosporine. Am J Gastroenterol 1998; 93: 442–8
Santos JV, Baudet JA, Casellas FJ, et al. Intravenous cyclosporine for steroid-refractory attacks of Crohn’s disease. Short-and long-term results. J Clin Gastroenterol 1995; 20: 207–10
Brynskov J, Freund L, Rasmussen SN, et al. A placebo-controlled, double-blind, randomized trial of cyclosporine therapy in active chronic Crohn’s disease. N Engl J Med 1989; 321: 845–50
Brynskov J, Freund L, Rasmussen S, et al. Final report on a placebo-controlled, double-blind, randomized, multicentre trial of cyclosporin treatment in active chronic Crohn’s disease. Scand J Gastroenterol 1991; 26: 689–95
Feagan BG, McDonald JW, Rochon J, et al. Low-dose cyclosporine for the treatment of Crohn’s disease. The Canadian Crohn’s Relapse Prevention Trial Investigators. N Engl J Med 1994; 330: 1846–51
Matsuda S, Koyasu S. Mechanisms of action of cyclosporine. Immunopharmacology 2000; 47: 119–25
Rovira P, Mascarell L, Truffa-Bachi P. The impact of immuno-suppressive drugs on the analysis of T cell activation. Curr Med Chem 2000; 7: 673–92
McMillan MA. Clinical pharmacokinetics of cyclosporin. Pharmacol Ther 1989; 42: 135–56
Fahr A. Cyclosporin clinical pharmacokinetics. Clin Pharmacokinet 1993; 24: 472–95
Faulds D, Goa KL, Benfield P. Cyclosporin. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in immunoregulatory disorders. Drugs 1993; 45: 953–1040
Noble S, Markham A. Cyclosporin. A review of the pharmacokinetic properties, clinical efficacy and tolerability of a microemulsion-based formulation (Neoral). Drugs 1995; 50: 924–41
Friman S, Backman L. A new microemulsion formulation of cyclosporin: pharmacokinetic and clinical features. Clin Pharmacokinet 1996; 30: 181–93
Kovarik JM, Mueller EA, van Bree JB, et al. Reduced inter- and intraindividual variability in cyclosporine pharmacokinetics from a microemulsion formulation. J Pharm Sci 1994; 83: 444–6
Browne BJ, Jordan S, Welsh MS, et al. Diet and cyclosporin A: pharmacokinetic comparison between Neoral and Sandimmune gelatin capsules. Transplant Proc 1994; 26: 2959–60
Wallemacq PE, Lhoest G, Latinne D, et al. Isolation, characterization and in vitro activity of human cyclosporin A metabolites. Transplant Proc 1989; 21: 906–10
Quesniaux VF. Pharmacology of cyclosporine (Sandimmune). III. Immunochemistry and monitoring. Pharmacol Rev 1990; 41: 249–58
Sandborn WJ, Strong RM, Forland SC, et al. The pharmacokinetics and colonic tissue concentrations of cyclosporine after i.v., oral, and enema administration. J Clin Pharmacol 1991; 31: 76–80
Brynskov J, Freund L, Campanini MC, et al. Cyclosporin pharmacokinetics after intravenous and oral administration in patients with Crohn’s disease. Scand J Gastroenterol 1992; 27: 961–7
Atkinson K, Britton K, Paull P, et al. Detrimental effect of intestinal disease on absorption of orally administered cyclosporine. Transplant Proc 1983; 15(snSuppl. 1): 2446–9
Kovarik JM, Koelle EU. Cyclosporin pharmacokinetics in the elderly. Drugs Aging 1999; 15: 197–205
Yee GC, McGuire TR. Pharmacokinetic drug interactions with cyclosporin (Part I). Clin Pharmacokinet 1990; 19: 319–32
Yee GC, McGuire TR. Pharmacokinetic drug interactions with cyclosporin (Part II). Clin Pharmacokinet 1990; 19: 400–15
Campana C, Regazzi MB, Buggia I, et al. Clinically significant drug interactions with cyclosporin. An update. Clin Pharmacokinet 1996; 30: 141–79
Hebert MF. Immunosuppressive agents. In: Levy RH, Thummel KE, Trager WF, et al., editors. Metabolic drug interactions. Philadelphia: Lippincott Williams & Wilkins, 2000: 499–510
Dresser GK, Spence JD, Bailey DG. Pharmacokinetic-pharmacodynamic consequences and clinical relevance of cytochrome P450 3A4 inhibition. Clin Pharmacokinet 2000; 38: 41–57
Ameer B, Weintraub RA. Drug interactions with grapefruit juice. Clin Pharmacokinet 1997; 33: 103–21
Michalets EL. Update: clinically significant cytochrome P-450 drug interactions. Pharmacotherapy 1998; 18: 84–112
Gomez DY, Wacher VJ, Tomlanovich SJ, et al. The effects of ketoconazole on the intestinal metabolism and bioavailability of cyclosporine. Clin Pharmacol Ther 1995; 58: 15–9
Greeson JM, Sanford B, Monti DA. St John’s wort (Hypericum perforatum): a review of the current pharmacological, toxilogical and clinical literature. Psychopharmacology 2001; 153: 402–14
Hebert MF, Roberts JP, Prueksaritanont T, et al. Bioavailability of cyclosporine with concomitant rifampin administration is markedly less than predicted by hepatic enzyme induction. Clin Pharmacol Ther 1992; 52: 453–7
MacDermott RP. Alterations in the mucosal immune system in ulcerative colitis and Crohn’s disease. Med Clin North Am 1994; 78: 1207–31
Bell SJ, Kamm MA. Review article: the clinical role of anti-TNFalpha antibody treatment in Crohn’s disease. Aliment Pharmacol Ther 2000; 14: 501–14
Breese EJ, Michie CA, Nicholls SW, et al. Tumor necrosis factor alpha-producing cells in the intestinal mucosa of children with inflammatory bowel disease. Gastroenterology 1994; 106: 1455–66
van Deventer SJ. Tumour necrosis factor and Crohn’s disease. Gut 1997; 40: 443–8
Brynskov J, Nielsen OH, Ahnfelt-Ronne I, et al. Cytokines (immunoinflammatory hormones) and their natural regulation in inflammatory bowel disease (Crohn’s disease and ulcerative colitis): a review. Dig Dis 1994; 12: 290–304
Noguchi M, Hiwatashi N, Liu Z, et al. Secretion imbalance between tumour necrosis factor and its inhibitor in inflammatory bowel disease. Gut 1998; 43: 203–9
Markham A, Lamb HM. Infliximab: a review of its use in the management of rheumatoid arthritis. Drugs 2000; 59: 1341–59
Wagner C, Mace K, DeWoody K, et al. Infliximab treatment benefits correlate with pharmacodynamic parameters in Crohn’s disease patients. Digestion 1998; 59(snSuppl. 3): 124–5
Sandborn WJ, Hanauer SB. Antitumor necrosis factor therapy for inflammatory bowel disease: a review of agents, pharmacology, clinical results, and safety. Inflamm Bowel Dis 1999; 5: 119–33
Kavanaugh A, St Clair EW, McCune WJ, et al. Chimeric antitumor necrosis factor-alpha monoclonal antibody treatment of patients with rheumatoid arthritis receiving methotrexate therapy. J Rheumatol 2000; 27: 841–50
Centocor Inc. Prescribing information for Remicade™ (infliximab) for iv injection. Malvern (PA): Centocor Inc., 1999 Nov
Rutgeerts P, D’Haens G, Targan S, et al. Efficacy and safety of retreatment with anti-tumor necrosis factor antibody (infliximab) to maintain remission in Crohn’s disease. Gastroenterology 1999; 117: 761–9
Maini RN, Breedveld FC, Kalden JR, et al. Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor alpha monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum 1998; 41: 1552–63
Papi C, Luchetti R, Gili L, et al. Budesonide in the treatment of Crohn’s disease: a meta-analysis. Aliment Pharmacol Ther 2000; 14: 1419–28
Christensen LA, Fallingborg J, Abildgaard K, et al. Topical and systemic availability of 5-aminosalicylate: comparison of three controlled release preparations in man. Aliment Pharmacol Ther 1990; 4: 523–33
Feagan BG, McDonald JWD. Medical therapy for inflammatory bowel disease. Curr Opin Gastroenterol 1997; 13: 307–11
Sartor RB. New therapeutic approaches to Crohn’s disease. N Engl J Med 2000; 342: 1664–6
Hanauer SB. Medical treatment for ulcerative colitis. Curr Opin Gastroenterol 2000; 16: 324–8
Stein RB, Hanauer SB. Medical therapy for inflammatory bowel disease. In: Lichtenstein GR, editor. Gastroenterology clinics of North America. Inflammatory bowel disease. Philadelphia: WB Saunders, 1999: 297–321
Sands BE. Therapy of inflammatory bowel disease. Gastroenterology 2000; 118 Suppl. 1: S68–82
Acknowledgements
This work was supported by the Robert Bosch Foundation, Stuttgart, Germany and the BMBF grant (FKZ 01 GG 9846). The secretarial help of Mrs S. Luginsland and Mrs H. Köhler is highly appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schwab, M., Klotz, U. Pharmacokinetic Considerations in the Treatment of Inflammatory Bowel Disease. Clin Pharmacokinet 40, 723–751 (2001). https://doi.org/10.2165/00003088-200140100-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-200140100-00003