Abstract
Use of an anatomical-physiological approach allows an investigator an alternative to regarding the whole body as a ‘black box’ producing biofluid specimens for drug assay, and then blindly applying a formula-driven mathematical approach to determine the pharmacokinetics and pharmacodynamics of the drug of interest. Instead, it means the investigator can consider that the body is the sum of interacting parts or regions connected anatomically by blood flow carrying the drug of interest, that the regions as well as the carrier blood are not homogeneous because each has a physiological role, and that the parts or regions are connected neurally and humorally so that the response in any region or part of the system may be modified by and/or modulate effects at another region or part. Such an approach is difficult to institute experimentally because a complicated (and often expensive) preparation is usually required in animal studies, and is rarely possible in research with humans because of ethical constraints.
Despite these restrictions, there are many examples of the use of an anatomical-physiological approach allowing greater insight into pharmacological problems than would have been possible with a conventional ‘whole body’ approach alone. This paper takes a number of examples from the discipline of anaesthesia and pain management and groups them to illustrate the principles of the approach regarding drug arteriovenous equality and tissue distribution, multiple sites of clearance and multiple sites of action.












Similar content being viewed by others
Notes
Distribution is more commonly used in pharmacokinetic literature and is used in this paper as the general term for the loss of drug from the circulating blood other than by elimination. Tissue uptake is the general term for referring to the drug gained by distribution into the tissues where the emphasis is placed on the consequences of drug being in the tissues. Redistribution is commonly used to denote loss from tissue back to blood, with or without ongoing distribution to other tissues.
Elimination is the irreversible removal of the drug from the body by all processes of excretion and metabolism.
Mean total body clearance is ‘mean’ because it is time-averaged, and is ‘total body’ because it is the sum of all regional clearances.
A ‘compartment’ is a virtual volume that is homogeneous in the distribution of its analyte-solute and may or may not correspond to tissues and regions defined anatomically and physiologically.
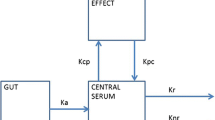
A region is defined as the tissue(s) between an afferent blood inflow and an efferent blood outflow; it may be heterogeneous or homogeneous with respect to tissue types or composition.
To be more precise, concentrations of drug in blood sampled from short arteries (e.g. coronary and bronchial) or proximally from long arteries would also differ temporally from those blood sampled distally from long arteries (e.g. femoral or brachial)with respect to rapidly changing solute concentration, but the differences would be negligible compared with any relevant arterio-venous pair.
Instantaneous rate of solute exchange (positive or negative, mass per unit time) between blood and tissue; equal to the (regional) clearance × arterial drug concentration.
Strictly, afferent-efferent to allow for gradients such as pulmonary arterial-to-arterial and portal venous-to-hepatic venous; usually arterio-venous, for which the drug extraction ratio is given by the concentration difference/afferent concentration.
References
Ludbrook GL, Upton RN, Grant C, et al. Acompartmental analysis of the pharmacokinetics of propofol in sheep. J Pharmacokinet Biopharm 1999; 27: 329–38
Upton RN, Ludbrook GL. A physiological model of induction of anaesthesia with propofol in sheep: 1. Structure and estimation of variables. Br J Anaesth 1997; 79: 497–504
Ludbrook GL, Upton RN. A physiological model of induction of anaesthesia with propofol in sheep: 2. Model analysis and implications for dose requirements. Br J Anaesth 1997; 79: 505–13
Bjorkman S, Akeson J, Nilsson F, et al. Ketamine and midazolam decrease cerebral blood flow and consequently their own rate of transport to the brain: an application of mass balance pharmacokinetics with a changing regional blood flow. J Pharmacokinet Biopharm 1992; 20: 637–52
Bjorkman S, Nilsson F, Akeson J, et al. The effect of thiopental on cerebral blood flow, and its relation to plasma concentration, during simulated induction of anaesthesia in a porcine model. Acta Anaesthesiol Scand 1994; 38: 473–8
Mather LE, Tucker GT, Pflug AE, et al. Meperidine kinetics in man: intravenous injection in surgical patients and volunteers. Clin Pharmacol Ther 1975; 17: 21–30
Upton RN, Ludbrook GL, Grant C, et al. Cardiac output is a determinant of the initial concentrations of propofol after short-infusion administration. Anesth Analg 1999; 89: 545–52
Fox IJ. History and developmental aspects of the indicator dilution technic. Circ Res 1962; 10: 381–92
Lassen NA, Perl W. Tracer kinetic methods in medical physiology. New York: Raven Press, 1979
Mather LE, Huang YF, Pryor ME, et al. Systemic and regional pharmacokinetics of bupivacaine and levobupivacaine in sheep. Anesth Analg 1998; 86: 805–11
Upton RN, Mather LE, Runciman WB, et al. The use of mass balance principles to describe regional drug distribution and elimination. J Pharmacokinet Biopharm 1988; 16: 13–29
Glass PS, Gan TJ, Howell S. A review of the pharmacokinetics and pharmacodynamics of remifentanil. Anesth Analg 1999; 89(4 Suppl.): S7–14
Fung HL, Sutton SC, Kamiya A. Blood vessel uptake and metabolism of organic nitrates in the rat. J Pharmacol Exp Ther 1984; 228: 334–41
Major E, Aun C, Yate PM, et al. Influence of sample site on blood concentrations of ICI 35868. Br J Anaesth 1983; 55: 371–5
Barratt RL, Graham GG, Torda TA. Kinetics of thiopentone in relation to the site of sampling. Br J Anaesth 1984; 56: 1385–91
Tucker GT, Mather LE. Pharmacology of local anaesthetic agents: pharmacokinetics of local anaesthetic agents. Br J Anaesth 1975; 47: 213–24
Hans P, Coussaert E, Cantraine F, et al. Predictive accuracy of continuous propofol infusions in neurosurgical patients: comparison of pharmacokinetic models. J Neurosurg Anesthesiol 1997; 9: 112–7
Riu PL, Riu G, Testa C, et al. Disposition of propofol between red blood cells, plasma, brain and cerebrospinal fluid in rabbits. Eur J Anaesthesiol 2000; 17: 18–22
Engdahl O, Abrahams M, Bjornsson A, et al. Cerebrospinal fluid concentrations of propofol during anaesthesia in humans. Br J Anaesth 1998; 81: 957–9
Dutta S, Matsumoto Y, Muramatsu A, et al. Steady-state propofol brain: plasma and brain: blood partition coefficients and the effect-site equilibration paradox. Br J Anaesth 1998; 81: 422–4
Rutten AJ, Mather LE, McLean CF. Cardiovascular effects and regional clearances of IV bupivacaine in sheep: enantiomeric analysis. Br J Anaesth 1991; 67: 247–56
Mather LE, Selby DG, Runciman WB. Effects of propofol and of thiopentone anaesthesia on the regional kinetics of pethidine in the sheep. Br J Anaesth 1990; 65: 365–72
Runciman WB, Mather LE, Ilsley AH, et al. Asheep preparation for studying interactions between blood flow and drug disposition. VI: effects of general or subarachnoid anaesthesia on blood flow and chlormethiazole disposition. Br J Anaesth 1986; 58: 1308–16
Upton RN, Mather LE, Runciman WB, et al. Uptake and elution of chlormethiazole, meperidine, and minaxolone in the hindquarters of sheep: implications for clearance calculations. J Pharm Sci 1991; 80: 108–12
Upton RN, Mather LE, Runciman WB. The in vitro uptake and metabolism of lignocaine, procainamide and pethidine by tissues of the hindquarters of sheep. Xenobiotica 1991; 21: 1–12
Upton RN, Runciman WB, Mather LE, et al. The uptake and elution of lignocaine and procainamide in the hindquarters of the sheep described using mass balance principles. J Pharmacokinet Biopharm 1988; 16: 31–40
Upton RN, Nancarrow C, McLean CF, et al. The in vivo blood, fat and muscle concentrations of lignocaine and bupivacaine in the hindquarters of sheep. Xenobiotica 1991; 21: 13–22
Milne RW, Sloan PA, McLean CF, et al. Disposition of morphine and its 3-glucuronide metabolites during morphine infusion in the sheep. Drug Metab Dispos 1993; 21: 1151–6
Milne RW, McLean CF, Mather LE, et al. Comparative disposition of morphine-3-glucuronide during separate intravenous infusions of morphine and morphine-3-glucuronide in sheep: importance of the kidney. Drug Metab Disp 1995; 23: 334–42
Milne RW, McLean CF, Mather LE, et al. Influence of renal failure of the disposition of morphine, morphine-3-glucuronide and morphine-6-glucuronide in sheep. J Pharm Exp Ther 1997; 282: 779–86
Mroszczak E, Combs D, Chaplin M, et al. Chiral kinetics and dynamics of ketorolac. J Clin Pharmacol 1996; 36: 521–39
Geisslinger G, Schaible HG. New insights into the site and mode of antinociceptive action of flurbiprofen enantiomers. J Clin Pharmacol 1996; 36: 513–20
Reilly CS, Merrell J, Wood AJ, et al. Comparison of the effects of isoflurane or fentanyl-nitrous oxide anaesthesia on propranolol disposition in dogs. Br J Anaesth 1988; 60: 791–6
Perry SM, Whelan E, Shay S, et al. Effect of i.v.anaesthesia with propofol on drug distribution and metabolism in the dog. Br J Anaesth 1991; 66: 66–72
Tucker GT, Mather LE, Lennard MS, et al. Plasma concentrations of the stereoisomers of prilocaine after administration of the racemate: implications for toxicity? Br J Anaesth 1990; 65: 333–6
Mather LE, Edwards SR, Duke CC, et al. Microdialysis study of the blood-brain equilibration of thiopentone enantiomers. Br J Anaesth 2000; 84: 67–73
Mather LE, Edwards SR, Duke CC. Electroencephalographic effects of thiopentone enantiomers in the rat: correlation with drug tissue distribution. Br J Pharmacol 1999; 128: 83–91
Mather LE. Edwards SR. Duke CC. Electroencephalographic effects of thiopentone and its enantiomers in the rat. Life Sci 2000; 66: 105–14
Chang DH, Ladd LA, Wilson KA, et al. Intravenous tolerability of large doses of levobupivacaine in sheep. Anesth Analg 2000; 91: 671–9
Huang YF, Pryor ME, Mather LE, et al. Cardiovascular and central nervous system effects of intravenous levobupivacaine and bupivacaine in sheep. Anesth Analg 1998; 86: 797–804
Chang DH, Ladd LA, Copeland S, et al. Direct cardiac effects of intracoronary levobupivacaine, bupivacaine and ropivacaine in the sheep. Br J Pharmacol. In press
Tucker GT, Mather LE. Properties, absorption and disposition of local anesthetics. In: Cousins MJ, Bridenbaugh PO, editors. Neural blockade. 3rd ed. Philadelphia (PA): Lippincott-Raven, 1998: 55–95
Bergmann NA. The legacy of John Snow: an appreciation of his life and scientific contribution on the 100th anniversary of his death. Anesthesiology 1957; 19: 595–606
Acknowledgements
The author acknowledges grant support from the National Health and Medical Research Council of Australia and from Ciroscience R&D Ltd, as well as the collaborative work with many colleagues, that went into providing the examples from the author’s personal collection of successful and unsuccessful experiments shown in this paper.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mather, L.E. Anatomical-Physiological Approaches in Pharmacokinetics and Pharmacodynamics. Clin Pharmacokinet 40, 707–722 (2001). https://doi.org/10.2165/00003088-200140100-00002
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-200140100-00002