Abstract
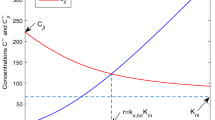
An intravenous loading dose is given to rapidly achieve a desired drug concentration in the blood. A loading dose calculated with the volume of distribution (Vd) at steady state will result in high peak concentrations and possibly serious adverse effects. A loading dose based on the central compartment Vd (Vc) followed by a maintenance infusion may also miss the target drug concentration and cause serious adverse effects. The Vd can be viewed as a time-dependent variable that expands from the Vc immediately after injection, to eventually include the steady-state Vd.
If the loading dose is calculated from a Vd determined after the time of peak effect (tmax), then the actual concentration will exceed the target concentration at the tmax. If a loading dose is calculated from a Vd before the peak effect occurs, the actual concentration will be insufficient to achieve the target concentration at tmax. A loading dose based on the Vd at the tmax will accurately achieve the concentration at the tmax without unexpected adverse effects.
To determine the Vd at peak effect, it is necessary that an effect can be measured, the peak effect can be detected and the plasma concentrations are sampled frequently enough to quantify the plasma concentrations at the tmax. For drugs that attain an ultra-fast effect (1 to 2 minutes), arterial samples need to be measured. If the onset of effect is intermediate or slow, venous blood can be sampled as the arterial and venous concentrations may be similar at the tmax.
Similar content being viewed by others
References
Schüttler J, Schwilden H, Stoeckel H. Pharmacokinetics as applied to total intravenous anaesthesia. Anaesthesia. 1983; 38S: 53–6.
Barratt RL, Graham GG, Torda TA. Kinetics of thiopentone in relation to the site of sampling. Br J Anaesth. 1984; 56: 1385–90.
Helmers H, Van Peer A, Woestenborghs R, et al. Alfentanil kinetics in the elderly. Clin Pharmacol Ther. 1984; 36: 239–43.
Lemmens HJM, Bovill JG, Burm AGL, et al. Alfentanil infusion in the elderly: prolonged computer infusion of alfentanil in the elderly surgical patient. Anaesthesia. 1988; 43: 850–6.
Chiou WL. The phenomenon and rationale of marked dependence of drug concentration on blood sampling site: implications in pharmacokinetics, pharmacodynamics, toxicology and therapeutics: I. Clin Pharmacokinet. 1989; 17: 175–99.
Chiou WL. The phenomenon and rationale of marked dependence of drug concentration on blood sampling site: implications in pharmacokinetics, pharmacodynamics, toxicology and therapeutics: II. Clin Pharmacokinet. 1989; 17: 275–90.
DiStefano JJ. Optimized blood sampling protocols and sequential design of kinetic experiments. Am J Physiol. 1981; 240: R259–65.
Ducharme J, Varin F, Bevan DR, et al. Importance of early blood sampling on vecuronium pharmacokinetic and pharmacodynamic parameters. Clin Pharmacokinet. 1993; 24: 507–18.
Raemer DB, Buschman A, Varvel JR, et al. The prospective use of population pharmacokinetics in a computer driven infusion system for alfentanil. Anesthesiology. 1990; 73: 66–72.
Shafer SL, Varvel JR, Aziz N, et al. Pharmacokinetics of fentanyl administered by computer-controlled infusion pump. Anesthesiology. 1990; 73: 1091–102.
Barvais L, Cantraine F, D’Hollander A, et al. Predictive accuracy of continuous alfentanil infusion in volunteers: variability of different pharmacokinetic sets. Anesth Analg. 1993; 77: 801–10.
Coetzee JF, Glen JB, Wium CA, et al. Pharmacokinetic model selection for target controlled infusions of propofol: assessment of three parameter sets. Anesthesiology. 1995; 82: 1328–45.
Vaughan DP, Tucker GT. General derivation of the ideal intravenous drug input required to achieve and maintain a constant plasma drug concentration: theoretical application to lignocaine. Eur J Clin Pharmacol. 1976; 10: 433–7.
Chiou WL. Potential pitfalls in the conventional pharmacokinetic studies: effects of the initial mixing of drug in blood and the pulmonary first-pass elimination. J Pharmacokinet Biopharm. 1979; 7: 527–36.
Roerig DL, Kotrly KJ, Dawson CA, et al. First-past uptake of verapamil, diazepam, and thiopental in the human lung. Anesth Analg. 1989; 69: 461–6.
Taeger K, Weninger E, Schmelzer F, et al. Pulmonary kinetics of fentanyl and alfentanil in surgical patients. Br J Anaesth. 1988; 61: 425–34.
Niazi S. Volume of distribution as a function of time. J Pharm Sci. 1976; 65: 452–4.
Shafer SL, Gregg KM. Algorithms to rapidly achieve and maintain stable drug concentrations at the site of drug effect with a computer-controlled infusion pump. J Pharmacokinet Biopharm. 1992; 20: 147–69.
Henthorn TK, Krejcie TC, Shanks CA, et al. Time-dependent distribution volume and kinetics of the pharmacodynamic effector site. J Pharm Sci. 1992; 81: 1136–8.
Minto CF, Schnider TW, Shafer SL. Pharmacokinetics and pharmacodynamics of remifentanil. II. Model application. Anesthesiology. 1997; 86: 24–33.
Minto CF, Schnider TW, Egan TD, et al. Influence of age and gender on the pharmacokinetics and pharmacodynamics of remifentanil. I. Model development. Anesthesiology. 1997; 86: 10–23.
Murphy MR, Hug CC. Pharmacokinetics of intravenous morphine in patients anesthetized with enflurane-nitrous oxide. Anesthesiology. 1981; 54: 187–92.
Scott JC, Stanski DR. Decreased fentanyl and alfentanil dose requirements with age: a simultaneous pharmacokinetic and pharmacodynamic evaluation. J Pharmacol Exp Ther. 1987; 240: 159–66.
Inturrisi CE, Colburn WA. Application of pharmacokineticpharmacodynamic modeling to analgesia. In: Foley KM, Inturrisi CE, editors. Advances in pain research and therapy: opioid analgesics in the management of clinical pain. New York: Raven Press, 1986: 441–52.
Rosow C. Pharmacology of opioid analgetic agents. In: Rogers MC, Tinker JH, Covino BG, et al., editors. Principles and practice of anesthesiology. StLouis: Mosby Year Book, 1993: 1155–83.
Hill HF, Chapman CR, Saeger LS, et al. Steady-state infusions of opioids in human. II. Concentration-effect relationships and therapeutic margins. Pain. 1990; 43: 69–79.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wada, D.R., Drover, D.R. & Lemmens, H.J.M. Determination of the Distribution Volume that can be Used to Calculate the Intravenous Loading Dose. Clin Pharmacokinet 35, 1–7 (1998). https://doi.org/10.2165/00003088-199835010-00001
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-199835010-00001