Summary
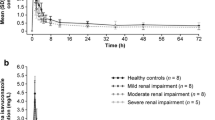
Grepafloxacin is mainly (approximately 90%) excreted by nonrenal mechanisms. The effect of renal impairment on the pharmacokinetics of grepafloxacin was evaluated in an open-label study involving 20 adults, 15 of whom had some degree of renal impairment (creatinine clearance 7.5 to 64.0 ml/min). Of these 15, 3 had mild renal impairment, 6 had moderate renal impairment, and 6 had severe renal impairment. Grepafloxacin 400mg was administered orally once daily for 7 days, and pharmacokinetic parameters were measured on days 1 and 7. The results show that both renal clearance and the amount of grepafloxacin excreted unchanged in urine, on day 1 and day 7, were significantly lower in individuals with severe renal impairment compared with those who were healthy. Renal clearance was 0.50 ± 0.05 ml/min/kg in healthy individuals vs 0.15 ± 0.05 ml/min/kg in patients with severe renal impairment on day 1, while the corresponding values on day 7 were 0.46 ± 0.04 ml/min/kg vs 0.14 ± 0.08 ml/min/kg, respectively. The percentage of grepafloxacin excreted unchanged in urine on day 1 was 5.1 ± 3.0 in the healthy individuals and 1.5 ± 0.7 in those with severe renal impairment. On day 7, the corresponding values were 7.9 ±1.9 and 2.9 ± 2.2. No other significant pharmacokinetic differences occurred between the 2 groups. Accumulation during multiple dose administration did not vary with the degree of renal impairment. We conclude that the pharmacokinetics of grepafloxacin are not significantly different in individuals with varying degrees of renal impairment. Hence, dose adjustment is not necessary during treatment of patients with renal dysfunction.
Similar content being viewed by others
References
St Peter WL, Redic-Kill KA, Halstenson CE. Clinical pharmacokinetics of antibiotics in patients with impaired renal function. Clin Pharmacokinet 1992; 22: 169–210
Schück O. Plasma protein binding of drugs and adjustment of their dosing regimen in patients with chronic renal failure. Int J Clin Pharmacol Ther Toxicol 1987; 25: 476–8
McNamee PT, Moore GW, McGeown MG, et al. Gastric emptying in chronic renal failure. BMJ 1985; 291: 310–1
Robson RA. Quinolone pharmacokinetics. Int J Antimicrob Agents 1992; 2: 3–10
Jungers P, Ganeval D, Hannedouche T, et al. Steady-state levels of pefloxacin and its metabolites in patients with severe renal impairment. Clin Pharmacol 1987; 33: 463–7
Blum RA, Schulz RW, Schentag JJ. Pharmacokinetics of lomefloxacin in renally compromised patients. Antimicrob Agents Chemother 1990; 34: 2364–8
Wolfson JS, Hooper DC. Pharmacokinetics of quinolones: newer aspects. Eur J Clin Microbiol Infect Dis 1991; 10: 267–74
Efthymiopoulos C, Bramer SL, Maroli A. Pharmacokinetics of grepafloxacin after oral administration of single and repeat doses in healthy young males. Clin Pharmacokinet 1997; 33 Suppl. 1: 1–8
Stein JM. Report 106-94-202. A study of the absorption, distribution, metabolism and excretion following oral administration of (14C) grepafloxacin to healthy human volunteers
Gibaldi M, Perrier D. Pharmacokinetics. 2nd ed. New York: Marcel Dekker, 1982
Lamp KC, Bailey EM, Rybak MJ. Ofloxacin clinical pharmacokinetics. Clin Pharmacokinet 1992; 22: 32–6
Bressolle F, Gonçalves F, Gouby A, et al. Pefloxacin clinical pharmacokinetics. Clin Pharmacokinet 1994; 27: 418–46
Vance-Bryan K, Guay DRP, Rotschafer JC. Clinical pharmacokinetics of ciprofloxacin. Clin Pharmacokinet 1990; 19: 434–61
Stuck AE, Kim DK, Frey FJ. Fleroxacin clinical pharmacokinetics. Clin Pharmacokinet 1992; 22: 116–31
Dudley MN. A review of the pharmacokinetic profile of temafloxacin. J Antimicrob Chemother 1991; 28 Suppl. C: 55–64
Burry RW, Becker GJ, Kincaid-Smith PS, et al. Elimination of enoxacin in renal disease. Clin Pharmacol Ther 1987; 41: 434–8
Efthymiopoulos C, Bramer SL, Maroli A. Effect of age and gender on the pharmacokinetics of grepafloxacin. Clin Pharmacokinet 1997; 33 Suppl. 1: 9–17
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Efthymiopoulos, C., Bramer, S.L., Maroli, A. et al. Effect of Renal Impairment on the Pharmacokinetics of Grepafloxacin. Clin-Pharmacokinet 33 (Suppl 1), 32–38 (1997). https://doi.org/10.2165/00003088-199700331-00007
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-199700331-00007