Summary
Thrombolytic agents are widely used for the treatment of acute thromboembolic diseases, especially acute myocardial infarction (AMI). These compounds include streptokinase, anistreplase, alteplase, urokinase and, although not commercially available yet, saruplase (prourokinase). The therapeutic window of these compounds is relatively small and subtherapeutic or toxic plasma concentrations may have serious clinical implications (insufficient thrombolysis, reocclusion and bleeding).
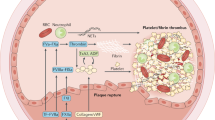
Among the factors that affect the pharmacokinetics and pharmacodynamics of thrombolytic agents, comedication is especially relevant since these drug interactions are partly predictable and sometimes preventable. Based on knowledge of the pharmacology of thrombolytic agents and general mechanisms by which pharmacokinetic drug interactions occur, interactions with alteplase and saruplase are expected. The clearance of alteplase is dependent on hepatic blood flow (HBF), and scientific evidence is emerging that saruplase is also a high-clearance compound. Each pharmacological agent that alters HBF and is given concurrently with one of these agents can change the plasma concentrations of those thrombolytics. Although there are no published data confirming druginduced changes in the metabolism of alteplase or saruplase by this mechanism in humans, indirect supportive evidence (clinical observations and animal experiments) is available.
An overview is presented of the anticipated effects of compounds that are frequently coadministered with thrombolytic agents on the pharmacokinetics of the thrombolytics with high-clearance properties. Since the clearance of these thrombolytics may be strongly affected by hypoperfusion of the liver as a result of cardiogenic haemodynamic failure, the role of circulatory changes in potential drug-drug interactions is also discussed.
Pharmacodynamic drug interactions are highly relevant in the treatment of acute thrombotic lesions and are still being evaluated to further optimise treatment strategies. As most of these treatments exist as combinations of thrombolytic, antithrombin and antiplatelet compounds, beneficial effects are partly offset by bleeding complications.
Changes in the pharmacokinetics and/or pharmacodynamics of thrombolytic agents may have serious consequences. It becomes imperative for the practising physician to be aware of benefits and risks of interactions with thrombolytic agents and especially of the fact that the principal way by which the pharmacokinetics of alteplase and, presumably, saruplase can be affected is by drug- and/or haemodynamic failure-induced changes of HBF.
Similar content being viewed by others
References
Sharma GVRK, Cella G, Parisi AF, et al. Thrombolytic therapy. N Engl J Med 1982; 306: 1268–76
ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17 187 cases of suspected acute myocardial infarction: ISIS-2. Lancet 1988; 2: 359–60
Verstraete M. Use of thrombolytic drugs in non-coronary disorders. Drugs 1989; 38: 801–21
Califf RM, Fortin DF, Tenaglia AN, et al. Clinical risks of thrombolytic therapy. Am J Cardiol 1992; 69: 12A–20A
Gonzalez ER, Jones LA, Ornato JP, et al. Adjunctive medications in patients receiving thrombolytic therapy: a multicenter prospective assessment. Ann Pharmacother 1992; 26: 1383–4
Granger CB, Califf RM, Topol EJ. Thrombolytic therapy for acute myocardial infarction: a review. Drugs 1992; 44: 293–325
Lew AS, Laramee P, Cereck B, et al. The hypotensive effect of intravenous streptokinase in patients with acute myocardial infarction. Circulation 1985; 72: 1321–6
Davies MC, Englert ME, De Renzo EC. Interaction of streptokinase and human plasminogen. I. Combining of streptokinase and plasminogen observed in the ultracentrifuge under a variety of experimental conditions. J Biol Chem 1964; 239: 2651–6
Gemmill JD, Hogg KJ, Burns JMA, et al. A comparison of the pharmacokinetic properties of streptokinase and anistreplase in acute myocardial infarction. Br J Clin Pharmacol 1991; 31: 143–7
Grierson DS, Bjornsson TD. Pharmacokinetics of streptokinase in patients based on amidolytic activator complex activity. Clin Pharmacol Ther 1987; 41: 304–13
Anderson JL. Development and evaluation of anisoylated plasminogen streptokinase activator complex (APSAC) as a second generation thrombolytic agent. J Am Coll Cardiol 1987; 10: 22B–27B
The GUSTO Investigators. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med 1993; 329: 673–82
Nilsson T, Wallen P, Mellbring G. In vivo metabolism of human tissue-type plasminogen activator. Scand J Haematol 1985; 33: 511–21
Seifried E, Tanswell P, Rijken DC, et al. Pharmacokinetics of antigen and activity of recombinant tissue-type plasminogen activator after infusion in healthy volunteers. Arzneimittel Forschung 1988; 38: 418–22
Bounameaux H, Stassen JM, Seghers C, et al. Influence of fibrin and liver blood flow on the turnover and systemic fibrinogenolytic effects of recombinant human tissue-type plasminogen activator in rabbits. Blood 1986; 67: 1493–7
De Boer A, Kluft C, Kasper FJ, et al. Liver blood flow as a major determinant of the clearance of recombinant tissue-type plasminogen activator. Thromb Haemost 1992; 67: 83–7
Van Griensven JMT, Burggraaf J, Huisman LG, et al. Liver blood flow affects the plasma concentrations of tissue-type plasminogen activator in patients with acute myocardial infarction [abstract]. Circulation 1994; 90: 1–553
De Boer A, Kluft C, Gerloff J, et al. Pharmacokinetics of saruplase, a recombinant unglycosylated human single-chain urokinase-type plasminogen activator and its effects on fibrinolytic and haemostatic parameters in healthy male subjects. Thromb Haemost 1993; 70: 320–5
Kounnas MZ, Henkin J, Argraves WS, et al. Low density lipoprotein receptor-related protein/α2-macroglobulin receptor mediates cellular uptake of pro-urokinase. J Biol Chem 1993; 268: 21862–7
Van Griensven JMT, Burggraaf J, Gerloff J, et al. The influence of changes in liver blood flow on the kinetics and dynamics of single chain urokinase-type plasminogen activator in healthy volunteers [abstract]. Br J Clin Pharmacol 1994; 37: 485P
Van Griensven JMT, Koster RW, Kroon R, et al. Liver blood flow and kinetics of pro-urokinase in acute myocardial infarction [abstract]. Circulation 1994; 90: I–553
Nies AS, Shand DG, Wilkinson GR. Altered hepatic blood flow and drug disposition. Clin Pharmacokinet 1976; 1: 135–55
Cohen AF, Burggraaf K, De Boer A, et al. Clearance of plasminogen activators — a major determinant of plasma concentration: therapeutic and diagnostic implications. Ann NY Acad Sci 1992; 667: 443–50
Velasco CE, Kerins DM, Howe D, et al. Effects of intravenous adenosine on tissue-type plasminogen activator pharmacokinetics in humans. Coron Artery Dis 1991; 2: 1069–75
De Boer A, Cohen AF, Kluft C, et al. Influence of heparin and a low molecular weight heparinoid on specific endogenous and exogenous fibrinolytic factors during rest and exercise. Thromb Haemost 1992; 68: 550–5
De Boer A, Kluft C, Kasper FJ, et al. Interaction study between nifedipine and recombinant tissue-type plasminogen activator. Br J Clin Pharmacol 1993; 36: 99–104
Walus KM, Fondacaro JD, Jacobson ED. Effects of adenosine and its derivatives on the canine intestinal vasculature. Gastroenterology 1981; 81: 327–34
Korbut R, Lidbury PS, Vane JR. Prolongation of fibrinolytic activity of tissue plasminogen activator by nitrovasodilators [letter]. Lancet 1990; 335: 669
Nicolini FA, Mehta JL, Nichols WW, et al. Prostacyclin analogue iloprost decreases thrombolytic potential of tissue-type plasminogen activator in canine coronary thrombosis. Circulation 1990; 81: 1115–22
Kerins DM, Shuh M, Kunitada S, et al. A prostacyclin analog impairs the response to tissue-type plasminogen activator during coronary thrombolysis: evidence for a pharmacokinetic interaction. J Pharmacol Exp Ther 1991; 257: 487–92
Svensson CK, Cumella JC, Tronolone M, et al. Effects of hydralazine, nitroglycerin, and food on estimated hepatic blood flow. Clin Pharmacol Ther 1985; 37: 464–8
Feely J. Nifedipine increases and glyceryl trinitrate decreases apparent liver blood flow in normal subjects. Br J Clin Pharmacol 1984; 17: 83–5
Hassan S, Pickles H. Epoprostenol (prostacyclin, PGI2) increases apparent liver blood flow in man. Prostaglandins Leukotrienes Med 1983; 10: 449–54
Selig RF, Kerr JC, Hobson RW, et al. Prostacyclin (epoprostenol): its effects on canine splanchnic blood flow during haemorrhagic shock. Arch Surg 1988; 116: 428–30
Topol EJ, Ellis SG, Califf RM, et al. Combined tissue-type plasminogen activator and prostacyclin therapy for acute myocardial infarction. J Am Coll Cardiol 1989; 14: 877–84
Bunting SR, Gryglewski R, Moncada AS, et al. Arterial walls generate from prostaglandin endoperoxides a substance (prostaglandin X) which relaxes strips of mesenteric and coeliac arteries and inhibits platelet aggregation. Prostaglandins 1976; 12: 897–913
Dinerman J, Mehta JL, Nichols WW. Systemic and coronary haemodynamic effects of prostacyclin and nitroprusside in conscious dogs. J Cardiovasc Pharm 1988; 12: 492–6
Smith EF, Gallenkamper W, Beckmann R, et al. Early and late administration of a PGI2-analogue, ZK 36374 (iloprost): effects on myocardial preservation, collateral blood flow and infarct size. Cardiovasc Res 1984; 18: 163–73
Gold HK, Johns JA, Leinbach RC, et al. Arandomized, blinded, placebo-controlled trial of recombinant human tissue-type plasminogen activator in patients with unstable angina pectoris. Circulation 1987; 75: 1192–9
Meredith PA, Elliott HL, Pasanini F, et al. Verapamil pharmacokinetics and apparent hepatic and renal blood flow. Br J Clin Pharmacol 1985; 20: 101–6
Meredith PA, Pasanini F, Elliott HL, et al. The effect of nisoldipine on apparent liver blood flow and effective renal plasma flow. Br J Clin Pharmacol 1985; 20: 235–7
Parker G, Ene MD, Daneshmend TK, et al. Do beta-blockers differ in their effects on hepatic microsomal enzymes and liver blood flow. J Clin Pharmacol 1984; 24: 493–9
Bauer LA, Stenwall M, Horn JR, et al. Changes in antipyrine and indocyanine green kinetics during nifedipine, verapamil, and diltiazem therapy. Clin Pharmacol Ther 1986; 40: 239–42
Bengtsson-Hasselgren B, Ronn O, Blychert L-O, et al. Acute effects of felodipine and nifedipine on hepatic and forearm blood flow in healthy men. Eur J Clin Pharmacol 1990; 38: 529–33
Branch RA, Shand DG, Nies AS. Regional haemodynamic effect of dopamine in the unanesthetized primate: failure to alter flow-dependent hepatic drug clearance. Eur J Pharmacol 1973; 24: 140–4
Richardson PDI, Withrington PG. Liver blood flow. II: effects of drugs and hormones on liver blood flow. Gastroenterology 1981; 81: 356–75
Bauer LA, Murray K, Horn JR, et al. Influence of nifedipine therapy on indocyanine green and oral propranolol pharmacokinetics. Eur J Clin Pharmacol 1989; 37: 257–60
Stenson RE, Constantino RT, Harrison DC. Interrelationships of hepatic blood flow, cardiac output, and blood levels of lidocaine in man. Circulation 1971; 43: 205–11
Zito RA, Reid PR. Lidocaine kinetics predicted by indocyanine green clearance. N Engl J Med 1978; 298: 1160–3
Fry ETA, Mack DL, Sobel BE. The nature of the synergy between tissue-type and single chain urokinase-type plasminogen activators. Thromb Haemost 1989; 62: 909–16
Rydzewski A, Takada Y, Takada A. Stimulation of plasmin catalyzed conversion of single-chain to two-chain urokinasetype plasminogen activator by sulfated polysaccharides. Thromb Haemost 1989; 62: 752–5
Collen D, Stump DC, Van de Werf F. Coronary thrombolysis in patients with acute myocardial infarction by intravenous infusion of synergistic thrombolytic agents. Am Heart J 1986; 112: 1083–4
ISIS-3 (Third International Study of Infarct Survival) Collaborative Group. A randomised comparison of streptokinase vs tissue plasminogen activator vs anistreplase and of aspirin plus heparin vs aspirin alone among 41 299 cases of suspected acute myocardial infarction. Lancet 1992; 339: 753–70
Basinski A, Naylor CD. Aspirin and fibrinolysis in acute myocardial infarction: meta-analytic evidence for synergy. J Clin Epidemiol 1991; 44: 1085–96
Webster MWI, Chesebro JH, Mruk JS. Antithrombotic therapy during and after thrombolysis for acute myocardial infarction. Coron Artery Dis 1990; 1: 190–8
Anderson HV, Willerson JT. Thrombolysis in acute myocardial infarction. N Engl J Med 1993; 329: 703–9
Aronson DL, Chang P, Kessler CM. Platelet-dependent thrombin generation after in vitro fibrinolytic treatment. Circulation 1992; 85: 1706–12
Grupo Italiano per lo Studio della Soprawivenza nell’Infarto miocardico. GISSI-3: effects of lisinopril and transdermal glyceryl trinitrate singly and together on 6-week mortality and ventricular function after acute myocardial infarction. Lancet 1994; 343: 1115–22
Kroon C, Ten Hove WR, De Boer A, et al. Highly variable anticoagulant response after subcutaneous administration of high dose (12,500 IU) heparin in patients with myocardial infarction and healthy volunteers. Circulation 1992; 86: 1370–5
de Bono DP, Simoons ML, Tijssen J, et al. Effect of early intravenous heparin on coronary patency, infarct size, and bleeding complications after alteplase thrombolysis: results of a randomised double blind European Cooperative Study Group trial. Br Heart J 1992; 67: 122–8
Tieffenbrunn AJ, Sobel BE. Thrombolysis and myocardial infarction. Fibrinolysis 1991; 5: 12–5
Fuster V. Coronary thrombolysis — a perspective for the practising physician. N Engl J Med. 1993; 329: 723–5
Collen D, Lijnen HR. Basic and clinical aspects of fibrinolysis and thrombolysis. Blood 1991; 78: 3114–24
Grupo Italiano per lo Studio della Soprawivenza nell’Infarto miocardico. GISSI-2: a factorial randomised trial of alteplase versus streptokinase and heparin versus no heparin among 12 490 patients with acute myocardial infarction. Lancet 1990; 336: 65–71
Topol EJ. Strategies for administration of tissue type plasminogen activator. Mol Biol Med 1991; 8: 219–34
Cannon CP, McCabe CH, Henry TD, et al. A pilot trial of recombinant desulfatohirudin compared with heparin in conjunction with tissue-type plasminogen activator and aspirin for acute myocardial infarction: results of the thrombolysis in myocardial infarction (TIMI) 5 trial. J Am Coll Cardiol 1994; 23: 993–1003
Woods KL, Fletcher F. Long-term outcome after intravenous magnesium sulphate in suspected acute myocardial infarction: the second Leicester intravenous magnesium intervention trial (LIMIT-2). Lancet 1994; 343: 816–9
Zahger D, Maaravi Y, Matzner Y, et al. Partial resistance to anticoagulation after streptokinase treatment for acute myocardial infarction. Am J Cardiol 1990; 66: 28–30
Kasper W, Hohnloser SH, Engler H, et al. Coronary reperfusion studies with pro-urokinase in acute myocardial infarction: evidence for synergism of low dose urokinase. J Am Coll Cardiol 1990; 16: 733–8
Collen D, Van de Werf F. Coronary arterial thrombolysis with low-dose synergistic combinations of recombinant tissuetype plasminogen activiator (rtPA) and recombinant singlechain urokinase-type plasminogen activator (rscu-PA) for acute mycocardial infarction. Am J Cardiol 1987; 60: 431–4
Collen D, Stassen J-M, Stump DC, et al. Synergism of thrombolytic agents in vivo. Circulation 1986; 74: 838–42
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
de Boer, A., van Griensven, J.M.T. Drug Interactions With Thrombolytic Agents. Clin. Pharmacokinet. 28, 315–326 (1995). https://doi.org/10.2165/00003088-199528040-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-199528040-00004