Summary
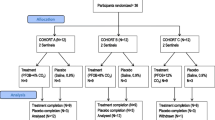
The optimal serum concentration of theophylline for the management of acute airways obstruction was evaluated by comparing the response to target concentrations at the extremes of the usual therapeutic range. 174 patients requiring intravenous theophylline were randomly assigned to a target concentration of 10 or 20 mg/L. Control of theophylline dosage using measured theophylline concentrations and evaluation of efficacy and toxicity was performed under double-blind conditions. 87 patients (50%) required hospital admission. Of these, 54 patients (62%) were followed throughout their hospital admission and reviewed at an outpatient clinic approximately 1 week after discharge. The duration of hospital stay, and rate and extent of improvement in peak expiratory flow rate were not different between the groups. There was significantly more toxicity in the 20 mg/L group. The initial target concentration for theophylline in the management of acute airway obstruction should be 10 mg/L under circumstances where concentration is used to control theophylline dosages.
Similar content being viewed by others
References
Chiou WL, Gadalla MAF, Peng GW. Method for the rapid estimation of the total body clearance and adjustment of dosage regimens in patients during a constant rate intravenous infusion. Journal of Pharmacokinetics and Biopharmaceutics 6: 135–151, 1978
Cohen J. Statistical power analysis for the behavioural sciences, 2nd ed., pp. 19–74, Lawrence Erlbaum Associates, Hillsdale, NJ, 1988
Coleridge J, Cameron P, Epstein J, Tiechtahl H. Intravenous aminophylline confers no benefit in acute asthma treated with intravenous steroids and inhaled bronchodilators. Australian and New Zealand Journal of Medicine 23: 348–355, 1993
Fairshter RD, Busse WW. Theophylline — how much is enough? Journal of Allergy and Clinical Immunology 77: 646–648, 1986
Holford NHG, Hashimoto Y, Sheiner LB. Time and theophylline concentration help explain the recovery of peak flow following acute airways obstruction: population analysis of a randomised concentration controlled trial. Clinical Pharmacokinetics 25: 506–515, 1993
Holford NHG, Sheiner LB. Understanding the dose-effect relationship: clinical application of pharmacokinetic-pharmacodynamic models. Clinical Pharmacokinetics 6: 429–453, 1981
Levine JH, Michael JR, Guarnieri T. Multifocal atrial tachycardia: a toxic effect of theophylline. Lancet 1: 12–14, 1985
Littenberg B, Gluck EH. A controlled trial of methylprednisolone in the emergency treatment of acute asthma. New England Journal of Medicine 314: 150–152, 1986
Mitenko PA, Ogilvie RI. Rational intravenous doses of theophylline. New England Journal of Medicine 289: 600–603, 1973
Murciano D, Auclair MH, Pariente R, Aubier M. A randomized controlled trial of theophylline in patients with severe chronic obstructive pulmonary disease. New England Journal of Medicine 320: 1521–1525, 1989
Powell JR, Vozeh S, Hopewell P, Costello J, Sheiner LB, et al. Theophylline disposition in acutely hospitalized patients: the effect of smoking, heart failure, severe airways obstruction, and pneumonia. American Review of Respiratory Disease 118: 229–238, 1978
Rice KL, Leatherman JW, Duane PG, Snyder LS, Harmon KR, et al. Aminophylline for acute exacerbations of chronic obstructive pulmonary disease. Annals of Internal Medicine 107: 305–309, 1987
Sanathanan LP, Peck CC. The randomized concentration-controlled clinical trial: An evaluation of its sample size efficiency. Controlled Clinical Trials 12: 780–794, 1991
Siegel D, Sheppard D, Gelb A, Weinberg PF. Aminophylline increases the toxicity but not the efficacy of an inhaled beta-adrenergic agonist in the treatment of acute exacerbations of asthma. American Review of Respiratory Disease 132: 283–286, 1985
Tinkelman DG, Moss BA, Bukantz SC, Sheffer AL, Dobken JH, et al. A multicenter trial of the prophylactic effect of ketotifen, theophylline, and placebo in atopic asthma. Journal Allergy and Clinical Immunology 76: 487–497, 1985
Vozeh S, Kewitz G, Perruchoud A, Tschan M, Kopp C, et al. Theophylline serum concentration and therapeutic effect in severe acute bronchial obstruction: the optimal use of intravenously administered theophylline. American Review of Respiratory Disease 125: 181–184, 1982
Wrenn K, Slovis CM, Murphy F, Greenberg RS. Aminophylline therapy for acute bronchospastic disease in the emergency room. Annals of Internal Medicine 115: 241–247, 1991
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Holford, N., Black, P., Couch, R. et al. Theophylline Target Concentration in Severe Airways Obstruction — 10 or 20 mg/L?. Clin. Pharmacokinet. 25, 495–505 (1993). https://doi.org/10.2165/00003088-199325060-00007
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-199325060-00007