Summary
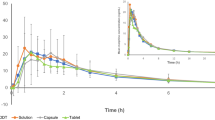
The pharmacokinetics of oral morphine sulphate as controlled release tablets (‘MS-Contin’) and solution were compared at steady-state. Plasma morphine concentrations were determined over 24 hours following the last dose of each drug when given in a randomised, crossover fashion to healthy subjects. Radioimmunoassay was used, which was sensitive yet provided good specificity relative to high-performance liquid chromatography. Controlled release tablets had 86% the bioavailability of the solution. Although each dose of controlled release tablets was double that of the solution, their peak plasma concentrations were the same. Time to maximum concentration was 3 times longer for controlled release tablets with an absorption half-life twice that of the solution. Elimination of both drugs was similar and biphasic with the minor terminal portion at 10 times the half-life of the major early process. These data explain the analgesic duration of 12 hours observed in clinical studies and the lack of accumulation with morphine compared with methadone.
Similar content being viewed by others
References
Berkowitz BA. The relationship of pharmacokinetics to pharmacological activity: morphine, methadone and naloxone. Clinical Pharmacokinetics 1: 219–230, 1976
Brunk SF, Delle M. Morphine metabolism in man. Clinical Pharmacology and Therapeutics 16: 51–57, 1974
Catlin DH. Pharmacokinetics of morphine by radioimmunoassay: the influence of immunochemical factors. Journal of Pharmacology and Experimental Therapeutics 200: 224–235, 1977
Ettinger DS, Vitale PJ, Trump DL. Important clinical pharmacologic considerations in the use of methadone in cancer patients. Cancer Treatment Reports 63: 457–459, 1979
Grabinski PY, Kaiko RF, Walsh TD, Foley KM, Houde RW. Morphine radioimmunoassay specificity before and after extraction of plasma and cerebrospinal fluid. Journal of Pharmaceutical Sciences 72: 27–29, 1983
Hanks GW, Rose NM, Aherne GW, Piall EM, Fairfield S, et al. Controlled-release morphine tablets: a double-blind trial in dental surgery patients. British Journal of Anaesthesia 53: 1259–1264, 1980
Homesley HD, Jobson VW, Welander C. Dosing range study of morphine sulfate continus (MS Contin) tablets in patients with chronic pain. American Journal of Clinical Oncology: Cancer Clinical Trials, in press, 1986
Khojasteh A, Evans W, Reynolds RD, Savarese JJ, Thomas G. Safety and efficacy of slow-release morphine sulphate tablets in cancer pain therapy. Proceedings of the American Society of Clinical Oncology 5: 256, 1986
Lapin J, Kaiko RF, Rogers AG, Wallenstein SL, Foley KM, et al. Cancer pain management with controlled-release oral morphine. 5th General Meeting of the American Pain Society, Dallas, October 18–20, 1985. Abstract no. CP37, p. 73, 1985
Leslie ST, Rhodes A, Black FM. Controlled release morphine sulphate tablets — a study in normal volunteers. British Journal of Clinical Pharmacology 9: 531–534, 1980
Matejczyk RJ, Kosinski MS, Kosinski JA. Determination of crossreactant drugs with a new morphine radioimmunoassay procedure. Journal of Forensic Sciences 30: 677–680, 1985
Meed SD, Kleinman P, Kantor TG, Blum RH. Use of MS Contin (slow-release morphine) in severe cancer related pain. 5th General Meeting of the American Pain Society, Dallas, October 18–20, 1985. Abstract no. P6, p. 22, 1985
Moore RA, Baldwin D, Allen MC, Watson PJ, Bullingham RE, McQuay HJ. Sensitive and specific morphine radioimmunoassay with iodine label: pharmacokinetics of morphine in man after intravenous administration. Annals of Clinical Biochemistry 21: 318–325, 1984
Nilsson MI, Meresaar U, Anggard E. Clinical pharmacokinetics of methadone. Acta Anaesthesiologica Scandinavica (Suppl. 74): 66–69, 1982
Säwe J. High-dose morphine and methadone in cancer patients: clinical pharmacokinetic considerations of oral treatment. Clinical Pharmacokinetics 11: 87–106, 1986
Säwe J, Dahlstrom B, Rane A. Steady-state kinetics and analgesic effect of oral morphine in cancer patients. European Journal of Clinical Pharmacology 24: 537–542, 1983
Stanski DR, Greenblatt DJ, Lowenstein E. Kinetics of intravenous and intramuscular morphine. Clinical Pharmacology and Therapeutics 24: 52–59, 1978
Svensson JO, Rane A, Säwe J, Sjoqvist F. Determination of morphine, morphine-3-glucuronide and (tentatively) morphine-6-glucuronide in plasma and urine using ion-pair high-performance liquid chromatography. Journal of Chromatography 230: 427–432, 1982
Vater M, Smith G, Aherne GW, Aitkenhead AR. Pharmacokinetics and analgesic effect of slow-release oral morphine sulphate in volunteers. British Journal of Anaesthesia 56: 821–827, 1984
Verebely K, Volavka J, Mule S, Resnick R. Methadone in man: pharmacokinetic and excretion studies in acute and chronic treatment. Clinical Pharmacology and Therapeutics 18: 180–190, 1975
Walsh TD. Clinical evaluation of slow-release morphine tablets. Advances in Pain Research and Therapy 9: 727–731, 1985
Welsh J, Stuart JFB, Habeshaw T, Blackie R, Whitehill D. A comparative pharmacokinetic study of morphine sulphate solution and MST Continus 30mg tablets in conditions expected to allow steady-state drug levels. In Stuart (Ed.) Methods of morphine estimation in biological fluids and the concept of free morphine, Royal Society of Medicine International Congress and Symposium Series No. 58, pp. 9–13, Academic Press, London, 1983
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Savarese, J.J., Goldenheim, P.D., Thomas, G.B. et al. Steady-State Pharmacokinetics of Controlled Release Oral Morphine Sulphate in Healthy Subjects. Clin-Pharmacokinet 11, 505–510 (1986). https://doi.org/10.2165/00003088-198611060-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-198611060-00006