Summary
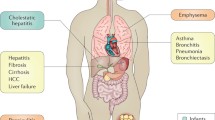
α1-Antitrypsin (α1AT) deficiency is the most common genetic cause of liver disease in children and emphysema in adults. Therapy for pulmonary disease attributable to α1AT deficiency includes α1AT augmentation therapy along with supportive measures. The α1AT preparation that is currently used for therapy is derived from fractionated plasma. The results of clinical trials suggest that augmentation therapy with α1AT slows the progression of emphysema and causes few adverse events. Patients with plasma levels of α1AT that are <11 μmol/L and who have airway obstruction should be considered for augmentation therapy. Novel approaches include the administration of aerosolised α1AT, recombinant α1AT, gene therapy and synthetic elastase inhibitors.
Similar content being viewed by others
References
Laurell C, Eriksson S. The electrophoretic α1-globulin pattern of serum in α1-antitrypsin deficiency. Scand J Clin Invest 1963; 15: 132–40
Morse JO. Alpha-1-antitrypsin deficiency (first of two parts). N Engl J Med 1978; 299(19): 1045–8
Morse JO. Alpha-1-antitrypsin deficiency (second of two parts). N Engl J Med 1978; 299(20): 1099–105
Kueppers F, Black L. α1-Antitrypsin and its deficiency. Am Rev Respir Dis 1974; 110: 176–94
Eriksson S. Pulmonary emphysema and alpha-1 antitrypsin deficiency. Acta Med Scand 1964; 175: 197–205
Hutchison D. Natural history of alpha-1-protease inhibitor deficiency. Am J Med 1988; 84: 3–12
Buist S, Burrows B, Cohen A, et al. Guidelines for the approach to the individual with severe hereditary alpha-1-antitrypsin deficiency: an official statement of the American Thoracic Society. Am Rev Respir Dis 1989; 140: 1494–7
Larsson C. Natural history and life expectancy in severe alpha1-antitrypsin deficiency, PiZ. Acta Med Scand 1978; 204: 345–51
Vogelmeier C, Hubbard R, Fells G, et al. Anti-neutrophil elastase defense of the normal human respiratory epithelial surface provided by the secretory leukoprotease inhibitor. J Clin Invest 1991; 87: 482–8
Nadziejko C, Finkelstein I, Balmes J. Contribution of secretory leukocyte proteinase inhibitor to the antiprotease defense system of the peripheral lung: effect of ozone-induced acute inflammation. Am J Respir Crit Care Med 1995; 152: 1592–8
Ohlsson K. Alpha1-antitrypsin and alpha2-macroglobulin: interactions with human neutrophil collagenase and elastase. Ann N Y Acad Sci 1975; 256: 409–19
Scharpe S, Eid M, Cooreman W, et al. Alpha-1-antitrypsin, an inhibitor of renin. Biochem J 1976; 153: 505–7
Brantly M, Nukiwa T, Crystal R. Molecular basis of α1-antitrypsin deficiency. Am J Med 1988; 84: 13–31
Travis J, Salvesen G. Human plasma proteinase inhibitors. Annu Rev Biochem 1983; 52: 655–709
Crystal R. The α1-antitrypsin gene and its deficiency states. Trends Genet 1989; 5: 411–7
Perlino E, Cortese R, Ciliberto G. The human alpha 1-antitrypsin gene is transcribed from two different promoters in macrophages and hepatocytes. EMBO J 1987; 6: 2767–71
Brantly M, Wittes J, Vogelmeier C, et al. Use of a highly purified α1-antitrypsin standard to establish ranges for the common normal and deficient α1-antitrypsin phenotypes. Chest 1991; 100: 703–8
Crystal R, Brantly M, Hubbard R, et al. The α1-antitrypsin gene and its mutations: clinical consequences and strategies for therapy. Chest 1989; 95: 196–208
Crystal R. α1-antitrypsin deficiency, emphysema, and liver disease: genetic basis and strategies for therapy. J Clin Invest 1990; 85: 1343–52
Carrell R, Jeppsson J, Laurell C, et al. Structure and variation of human α1-antitrypsin. Nature 1982; 298: 329–34
Long G, Chandra I, Woo S, et al. Complete sequence of the cDNA for human α1-antitrypsin and the gene for the S variant. Biochemistry 1984; 23: 4828–37
Mega T, Lujan E, Yoshida A. Studies on the oligosaccharide chains of human α1-protease inhibitor: I. Isolation of glycopeptides. J Biol Chem 1980; 255: 4053–6
Potempa J, Korzus E, Travis J. The serpin superfamily of proteinase inhibitors: structure, function and regulation. J Biol Chem 1994; 269: 1557–60
Lomas D. New insights into the structural basis of α1-antitrypsin deficiency. Q J Med 1996; 89: 807–12
Huber R, Carrell R. Implications of the three-dimensional structure of α1-antitrypsin for structure and function of serpins. Biochemistry 1989; 28: 8951–66
Carrell R, Evans D, Stein P. Mobile reactive centre of serpins and the control of thrombosis. Nature 1991; 353: 576–8
Loebermann H, Tokuoka R, Deisenhofer J, et al. Human α1-proteinase inhibitor crystal structure analysis of two crystal modifications, molecular model and preliminary analysis of the implications for function. J Mol Biol 1984; 177: 531–56
Johnson D, Travis J. The oxidative inactivation of human α1-proteinase inhibitor — further evidence for methionine at the reactive center. J Biol Chem 1979; 254: 4022–6
Beatty K, Matheson N, Travis J. Kinetic and chemical evidence for the inability of oxidized alpha-1-proteinase inhibitor to protect lung elastin from elastolytic degradation. Hoppe Seylers Z Physiol Chem 1984; 365: 731–6
Hubbard R, Ogushi F, Fells G, et al. Oxidants spontaneously released by alveolar macrophages of cigarette smokers can inactivate the active site of α1-antitrypsin, rendering it ineffective as an inhibitor of neutrophil elastase. J Clin Invest 1987; 80: 1289–95
Janoff A. Elastase and emphysema: current assessment of the protease-antiprotease hypothesis. Am Rev Respir Dis 1985; 132: 417–33
Bruun-Petersen K, Bruun-Petersen G, Dahl R, et al. Alpha-1-antitrypsin alleles in patients with pulmonary emphysema, detected by DNA amplification (PCR) and oligonucleotide probes. Eur Respir J 1992; 5: 531–7
Elliott P, Lomas D, Carrell R, et al. Inhibitory conformation of the reactive loop of α1-antitrypsin. Nat Struct Biol 1996; 3: 676–81
Wright H, Scarsdale J. Structural basis for serpin inhibitor activity. Proteins 1995; 22: 210–25
Carrell R, Lomas D, Sidhar S, et al. α1-Antitrypsin deficiency: a conformational disease. Chest 1996; 110: S243–7
Lieberman J, Gaidulis L, Garoutte B, et al. Identification and characteristics of the common alpha-1-antitrypsin phenotypes. Chest 1972; 62: 557–64
Brantly M, Paul L, Miller B, et al. Clinical features and natural history of the destructive lung disease associated with alpha-1-antitrypsin deficiency of adults associated with pulmonary symptoms. Am Rev Respir Dis 1988; 138: 327–36
Allen R, Harley R, Talamo R. A new method for determination of alpha-1-antitrypsin phenotypes using isoelectric focusing on polyacrylamide gel slabs. Am J Clin Pathol 1974; 62: 732–9
Jeppsson J. Amino acid substitution Glu → Lys in α1-antitrypsin PiZ. FEBS Lett 1976; 65: 195–7
Yoshida L, Lieberman L, Gaidulis L, et al. Molecular abnormality of human alpha-1-antitrypsin variant (PiZ) associated with plasma activity deficiency. Proc Acad Sci 1976; 73: 1324–30
Jeppson J, Larsson C, Eriksson S. Characterization of A-1-antitrypsin deficiency. N Engl J Med 1975; 293: 576–81
Snider G, Lucey E, Stone P. State of the art: animal models of emphysema. Am Rev Respir Dis 1986; 133: 149–69
Gishen P, Saunders A, Tobin M, et al. Alpha-1-antitrypsin deficiency: the radiological features of pulmonary emphysema in subjects of Pi type Z and Pi type SZ: a survey of the British Thoracic Association. Clin Radiol 1982; 33: 371–7
Silverman E, Pierce J, Province M, et al. Variability of pulmonary function in alpha-1-antitrypsin deficiency: clinical correlates. Ann Intern Med 1989; 111: 982–91
Owen M, Brennan S, Lewis J, et al. Mutation of antitrypsin to antithrombin: α1-antitrypsin Pittsburgh (358 Met — Arg), a fatal bleeding disorder. N Engl J Med 1983; 309: 694–8
Janoff A, Sloan B, Weinbaum G, et al. Experimental emphysema induced with purified human neutrophil elastase: tissue localization of the instilled protease. Am Rev Respir Dis 1977; 115: 461–78
Senior R, Tegner H, Kuhn C, et al. The induction of pulmonary emphysema with human leukocyte elastase. Am Rev Respir Dis 1977; 116: 469–75
Snider G, Lucey E, Christensen T, et al. Emphysema and bronchial secretory cell metaplasia induced in hamsters by human neutrophil products. Am Rev Respir Dis 1984; 129: 155–60
Snider G, Sherter C, Koo K, et al. Respiratory mechanics in hamsters following treatment with endotracheal elastase or collagenase. J Appl Physiol 1977; 42: 206–15
Karlinsky J, Fredette J, Davidovits G, et al. The balance of lung connective tissue elements in elastase-induced emphysema. J Lab Clin Med 1983; 102: 151–62
Kuhn C, Yu S, Chraplyvy M, et al. The induction of emphysema with elastase: II. Changes in connective tissue. Lab Invest 1976; 34: 372–80
Gadek J, Zimmerman R, Fells G, et al. Antielastases of the human alveolar structures: implications for the protease-antiprotease theory of emphysema. J Clin Invest 1981; 68: 889–98
Beatty K, Bieth J, Travis J. Kinetics of association of serine proteinases with native and oxidized α-a-proteinase inhibitor and α-1-antichymotrypsin. J Biol Chem 1980; 55: 3931–4
Rao N, Wehner N, Marshall B, et al. Proteinase-3 (Pr-3): a polymorphonuclear leukocyte serine proteinase. Ann N Y Acad Sci 1991; 624: 60–8
Hubbard R, Crystal R. Alpha 1-antitrypsin augmentation therapy for alpha 1-antitrypsin deficiency. Am J Med 1988; 84: 52–62
Fujita J, Nelson N, Daughton D, et al. Evaluation of elastase vs. anti-elastase balance in patients with chronic bronchitis and pulmonary emphysema. Am Rev Respir Dis 1991; 142: 57–62
Galdston M, Melnick E, Goldring R, et al. Interactions of neutrophil elastase, serum trypsin inhibitory activity, and smoking history as risk factors for chronic obstructive pulmonary disease in patients with MM, MZ, and ZZ phenotypes for alpha1-antitrypsin. Am Rev Respir Dis 1984; 116: 837–46
Abboud R, Rushton J, Grzybowski S. Interrelationship between neutrophil elastase, serum alpha1-antitrypsin, lung function and chest radiography in patients with chronic airflow obstruction. Am Rev Respir Dis 1979; 120: 31–40
Lam S, Abboud R, Chan-Yeung M, et al. Neutrophil elastase and pulmonary function in subjects with intermediate alpha1-antitrypsin deficiency (MZ phenotype). Am Rev Respir Dis 1979; 119: 941–51
Rodriguez J, Seal J, Radin A, et al. Neutrophil lysosomal elastase activity in normal subjects and in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1979; 119: 409–17
Kramps J, Bakker W, Dijkman J. A matched-pair study of the leukocyte elastase-like activity in normal persons and in emphysematous patients with and without alpha1-antitrypsin deficiency. Am Rev Respir Dis 1980; 121: 253–61
Snider G. Emphysema: the first two centuries and beyond. A historical overview, with suggestions for future research: part 2. Am Rev Respir Dis 1992; 146: 1615–22
Eriksson S. Studies in α1-antitrypsin deficiency. Acta Med Scand 1965; 177: 1–85
Tobin M, Cook P, Hutchison D. Alpha-1-antitrypsin deficiency: the clinical and physiological features of pulmonary emphysema in subjects homozygous for Pi type Z. A survey by the British Thoracic Association. Br J Dis Chest 1983; 77: 14–27
Buist A, Burrows B, Eriksson S, et al. The natural history of air-flow obstruction in PiZ emphysema. Am Rev Respir Dis 1983; 127: S43–5
Janus E, Phillips N, Carrell R. Smoking, lung function, and alpha-1-antitrypsin deficiency. Lancet 1985; I: 152–4
Silverman E, Province M, Rao D, et al. A family study of the variability of pulmonary function in α1-antitrypsin deficiency. Am Rev Respir Dis 1990; 142: 1015–21
Poller W, Faber J, Olek K. Highly variable clinical course in severe α1-antitrypsin deficiency — use of polymerase chain reaction for the detection of rare deficiency alleles. Klin Wochenschr 1990; 68: 857–63
Blank C, Brantly M. Clinical features and molecular characteristics of α1-antitrypsin deficiency. Ann Allergy 1994; 72: 105–22
Gaillard M, Kilroe-Smith T, Nogueira C, et al. Alpha-1-protease inhibitor in bronchial asthma: phenotypes and biochemical characteristics. Am Rev Respir Dis 1992; 145: 1311–5
Evald T, Dirksen A, Keittelman S, et al. Decline in pulmonary function in patients with α1-antitrypsin deficiency. Lung 1990; 168: S579–85
Burrows B, Knudsen R, Camilli A, et al. The ‘horse-racing effect’ and predicting decline in forced expiratory volume in one second from screening spirometry. Am Rev Respir Dis 1987; 135: 788–93
Seersholm N, Kok-Jensen A, Dirksen A. Decline in FEV1 among patients with severe hereditary α1-antitrypsin deficiency type PiZ. Am J Respir Crit Care Med 1995; 152: 1922–5
Seersholm N, Kok-Jensen A. Survival in relation to lung function and smoking cessation in patients with severe hereditary alpha-1-antitryspin deficiency. Am J Respir Crit Care Med 1995; 151: 369–73
Anthonisen N, Connett J, Kiley J, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1: the Lung Health Study. JAMA 1994; 272: 1497–505
MRC Working Party. Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Lancet 1981; I: 681–6
American Thoracic Society Statement. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1995; 152: S77–120
ERS-Consensus Statement. Optimal assessment and management of chronic obstructive pulmonary disease (COPD). Eur Respir J 1995; 8: 1398–420
Hay J, Robin E. Cost-effectiveness of alpha-1-antitrypsin replacement therapy in treatment of congenital chronic obstructive pulmonary disease. Am J Public Health 1991; 81: 427–33
Gadek J, Klein H, Holland P, et al. Replacement therapy of alpha 1-antitrypsin deficiency: reversal of protease-antiprotease imbalance within the alveolar structures of PiZZ subjects. J Clin Invest 1981; 68: 1158–65
Wewers M, Casolaro M, Sellers S, et al. Replacement therapy for alpha1-antitrypsin deficiency associated with emphysema. N Engl J Med 1987; 316: 1055–62
Jones E, Vergala J, Steer C, et al. Metabolism of intact and desialylated alpha1-antitrypsin. Clin Sci Mol Med 1978; 55: 139–48
Eriksson S. Replacement therapy in alpha1-antitrypsin deficiency. J Intern Med 1989; 225: 69–72
Hubbard R, Sellers S, Czerski D, et al. Biochemical efficacy and safety of monthly augmentation therapy for α1-antitrypsin deficiency. JAMA 1988; 260: 1259–64
Burnouf T, Constans J, Clerc A, et al. Biochemical and biological properties of an alpha-1-antitrypsin concentrate. Vox Sang 1987; 52: 291–7
Coan M. Purification of alpha-1-proteinase inhibitor: preparation and properties of a therapeutic concentrate. Am J Med 1988; 84: S32–6
Constans J, Carles P, Boneu A, et al. Clinical pharmacokinetics of alpha-1-antitrypsin in homozygous PiZ deficient patients. Clin Pharmacokinet 1992; 23: 161–8
Schmidt E, Rasche B, Ulmer W, et al. Replacement therapy for alpha-1-protease inhibitor deficiency in PiZ subjects with chronic obstructive lung disease. Am J Med 1988; 84: 63–9
Ulmer W, Schmidt E, Rasche B. Long term effect on lung function of alpha-1-protease inhibitor substitution therapy in COPD patients with PiZZ phenotype. Eur Respir J 1990; 3: S21–2
Barker A, Siemsen F, Pasley D, et al. Replacement therapy for hereditary alpha-1-antitrypsin deficiency: a program for long-term administration. Chest 1994; 105: 1406–10
Schwaiblmair M, Vogelmeier C, Fruhmann G. Long-term augmentation therapy in twenty patients with severe alpha-1-antitrypsin deficiency — three-year follow up. Respiration 1997; 64: 10–5
Crystal R, editor. Alpha-1-antitrypsin deficiency. New York: M. Dekker, 1996
Eriksson S, Wu M. Aspects of treatment in alpha-1-antitrypsin deficiency: insights derived from a Swedish PiZZ series. Eur Respir J 1990; 3Suppl. 9: S39–43
Hutchison D, Hughes M. Alpha-1-antitrypsin replacement therapy: will its efficacy ever be proved? Eur Respir J 1997; 10: 2191–3
Seersholm N, Wencker M, Banik N, et al. Does α1-antitrypsin augmentation therapy slow the annual decline in FEV1 in patients with severe hereditary α1-antitrypsin deficiency? Eur Respir J 1997; 10: 2260–3
Wencker M, Banik N, Buhl R, et al., for the Wissenschaftliche Arbeitsgemeinschaft zur Therapie von Lungenerkrankungen (WATL) — α1AT Study Group. Long-term treatment of α1-antitrypsin deficiency-related pulmonary emphysema with human α1-antitrypsin. Eur Respir J 1998; 11: 428–33
Physicians’ Desk Reference. 49th ed. Montvale: Medical Economics Data Production Company, 1995: 1708–10
Hubbard R, Brantly M, Sellers S, et al. Delivery of proteins for therapeutic purposes by aerosolization: direct augmentation of anti-neutrophil elastase defenses of the lower respiratory tract in deficiency with an aerosol of α1-antitrypsin. Ann Intern Med 1989; 111: 206–12
Smith R, Traber L, Traber D, et al. Pulmonary deposition and clearance of aerosolized alpha-1-antitrypsin inhibitor administered to dogs and sheep. J Clin Invest 1989; 84: 1145–54
Hubbard R, McElvaney N, Sellers S, et al. Recombinant DNA-produced alpha-1-antitrypsin administered by aerosol augments lower respiratory tract neutrophil anti-elastase in individuals with alpha-1-antitrypsin deficiency. J Clin Invest 1989; 84: 1349–54
Birrer P, McElvaney N, Gillissen A, et al. Intravenous administration of recombinant secretory leukoprotease inhibitor as strategy to augment antineutrophil elastase protective screen of the lung. J Appl Physiol 1992; 317–23
Albertson T, Walby W, Allen R, et al. The pharmacology and toxicology of three new biologic agents used in pulmonary medicine. Clin Toxicol 1995; 33: 427–38
Cherfas J. Sheep to produce α1-antitrypsin. BMJ 1992; 304: 527
Straus S, Fells G, Wewers M, et al. Evaluation of recombinant DNA-directed E. coli produced alpha-1-antitrypsin as an anti-neutrophil elastase for potential replacement therapy of alpha-1-antitrypsin deficiency. Biochem Biophys Res Comm 1985; 130: 1177–84
Casolaro M, Fells G, Wewers M, et al. Augmentation of lung antineutrophil elastase capacity with recombinant human alpha-1-antitrypsin. J Appl Physiol 1987; 63: 2015–23
Mast A, Salvesen G, Schnebli H, et al. Evaluation of the rapid plasma elimination of recombinant alpha-1-proteinase inhibitor: synthesis of polyethylene glycol conjugates with improved therapeutic potential. J Lab Clin Med 1990; 116: 58–65
Kay M, Baley P, Rothenberg S, et al. Expression of human α1-antitrypsin in dogs after autologous transplantation of retroviral transduced hepatocytes. Proc Natl Acad Sci U S A 1992; 89: 89–93
Jaffe H, Danel C, Longenecker G, et al. Adenovirus-mediated in vivo gene transfer and expression in normal rat liver. Nat Genet 1992; 1: 372–8
Garver R, Chytil A, Courtney M, et al. Clonal gene therapy: transplanted mouse fibroblast clones express human α1-antitrypsin gene in vivo. Science 1987; 237: 762–4
Crystal R. Protocol for gene therapy of the respiratory manifestations of cystic fibrosis using a replication deficient recombinant adenovirus to transfer the normal cystic fibrosis transmembrane conductance regulator cDNA to the airway epithelium. Fed Regist 1992; 58: 21737–8
Knoell D, Wewers M. Clinical implications of gene therapy for alpha-1-antitrypsin deficiency. Chest 1995; 107: 535–45
Setoguchi Y, Jaffe H, Chu C, et al. Intraperitoneal in vivo gene therapy to deliver α1-antitrypsin to the systemic circulation. Am J Respir Cell Mol Biol 1994; 10: 369–77
Rosenfeld M, Siegfried W, Yoshimura K, et al. Adenovirus-mediated transfer of a recombinant α1-antitrypsin gene to the lung epithelium in vivo. Science 1991; 252: 431–4
Crystal R. Gene therapy strategies for pulmonary disease. Am J Med 1992; 92Suppl. 6A: S44–52
Esquivel C, Vicente E, Van Thiel D, et al. Orthoptic liver transplantation for alpha-1-antitrypsin deficiency: an experience in 29 children and 10 adults. Transplant Proc 1987; 19: 3798–802
Wewers M, Gadek J, Keogh B, et al. Evaluation of danazol therapy for patients with PiZZ alpha-1-antitrypsin deficiency. Am Rev Respir Dis 1986; 134: 476–80
Wewers M, Brantly M, Casolaro M, et al. Evaluation of tamoxifen as a therapy to augment alpha-1-antitrypsin concentrations in Z homozygous alpha-1-antitrypsin deficient individuals. Am Rev Respir Dis 1987; 135: 401–2
Powers J. Synthetic elastase inhibitors: prospects for use in the treatment of emphysema. Am Rev Respir Dis 1983; 127: S54–8
Trainer A. Synthetic inhibitors of human neutrophil elastase. Trends Pharmacol Sci 1987; 8: 303–7
Groutas W. Inhibitors of leukocyte elastase and leukocyte cathepsin G: agents for the treatment of emphysema and related ailments. Med Res Rev 1987; 7: 227–41
Edwards P, Berstein P. Synthetic inhibitors of elastase. Med Res Rev 1994; 14: 127–94
Powers J, Plaskon R, Chih-Min K. Low-molecular-weight inhibitors of neutrophil elastase. In: Crystal R, editor. Alpha-1-antitrypsin deficiency. New York: M. Dekker, 1996: 341–70
Zimmerman M, Morman H, Mulvey D, et al. Inhibition of elastase and other serine proteases by heterocyclic acylating agents. J Biol Chem 1980; 255: 9848–51
Doherty J, Ashe B, Argenbright L, et al. Cephalosporin antibiotics can be modified to inhibit human leukocyte elastase. Nature 1986; 322: 192–4
Williams J, Falcone R, Knee C, et al. Biologic characterization of ICI 200,880 and ICI 200,355, novel inhibitors of human neutrophil elastase. Am Rev Respir Dis 1991; 144: 875–83
Doherty J, Shah S, Finke P, et al. Chemical, biochemical, pharmacokinetic, and biological properties of L-680,833: a potent, orally active monocyclic β-lactam inhibitor of human polymorphonuclear leukocyte elastase. Proc Natl Acad Sci U S A 1993; 90: 8727–31
Weinmann G, Hyatt R. Evaluation and research in lung volume reduction surgery. Am J Respir Crit Care Med 1996; 154: 1913–8
Yusen R, Lefrak S, Trulock E. Evaluation and preoperative management of lung volume reduction surgery candidates. Clin Chest Med 1997; 18: 199–224
Sciurba F. Early and long-term functional outcomes following lung volume reduction surgery. Clin Chest Med 1997; 18: 259–76
Patterson G, Cooper J, Goldman B, et al. Technique of successful clinical double-lung transplantation. Ann Thorac Surg 1988; 45: 626–33
Marshall S, Kramer M, Lewiston N, et al. Selection and evaluation of recipients for heart-lung and lung transplantation. Chest 1990; 98: 1488–94
Trulock E. Recipient selection. Chest Surg Clin N Am 1993; 3: 1–18
Mal H, Andreassian B, Pamela F, et al. Unilateral lung transplantation in end-stage pulmonary emphysema. Am Rev Respir Dis 1989; 140: 797–802
Mal H, Sleiman C, Jebrak G, et al. Functional results of single-lung transplantation for chronic obstructive lung disease. Am J Respir Crit Care Med 1994; 149: 1476–81
Levine S, Anzueto A, Peters J, et al. Medium term functional results of single-lung transplantation for endstage obstructive lung disease. Am J Respir Crit Care Med 1994; 150: 398–402
Brunsting L, Lupinetti F, Cascade P, et al. Pulmonary function in single lung transplantation for chronic obstructive pulmonary disease. J Thorac Cardiovasc Surg 1994; 107: 1337–45
Cooper J, Patterson G, Grossman R, et al. Double-lung transplant for advanced chronic obstructive lung disease. Am Rev Respir Dis 1989; 139: 303–7
Trulock E, Egan T, Kouchoukos N, et al. Single lung transplantation for severe chronic obstructive pulmonary disease. Chest 1989; 96: 738–42
Trulock E, Cooper J, Kaiser L, et al. The Washington University-Barnes Hospital experience with lung transplantation. JAMA 1991; 266: 1943–6
Patterson G, Maurer J, Williams T, et al. Comparison of outcomes of double and single lung transplantation for obstructive lung disease. J Thorac Cardiovasc Surg 1991; 101: 623–32
Levy R, Ernst P, Levine S, et al. Exercise performance after lung transplantation. J Heart Lung Transplant 1993; 12: 27–33
Howard D, Iademarco E, Trulock E. The role of cardiopulmonary exercise testing in lung and heart-lung transplantation. Clin Chest Med 1994; 15: 405–20
Hosenpud J, Novick R, Breen T, et al. The Registry of the International Society for Heart and Lung Transplantation: twelfth official report — 1995. J Heart Lung Transplant 1995; 14: 805–15
King M, Campbell E, Gray B, et al. The proteinase-antiproteinase balance in alpha-1-proteinase inhibitor-deficient lung transplant recipients. Am J Respir Crit Care Med 1994; 149: 966–71
Trulock E. Lung transplantation for α1-antitrypsin deficiency emphysema. Chest 1996; 110: S248–94
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schwaiblmair, M., Vogelmeier, C. α1-Antitrypsin. Drugs & Aging 12, 429–440 (1998). https://doi.org/10.2165/00002512-199812060-00002
Published:
Issue Date:
DOI: https://doi.org/10.2165/00002512-199812060-00002