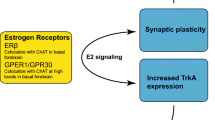
Summary
Estrogen replacement therapy appears to have significant beneficial effects on cognition and mood in the elderly. In recent studies, its use has been associated with short term symptomatic cognitive improvement and with a decreased risk of (or a delay in) developing Alzheimer’s disease (AD). Clinical reports are supported by substantial basic scientific evidence of the neuroprotective effects of estrogens. Their specific effects on dementia and cognitive impairment remain to be delineated. Ongoing randomised trials in AD will only provide information on the symptomatic effects of estrogens. Although basic research will progress, there is currently sufficient knowledge to promote active clinical research on the possible disease-modifying or neuroprotective effects of estrogens in the elderly.
Similar content being viewed by others
References
Belchetz PE. Hormonal treatment of postmenopausal women. N Engl J Med 1994; 330: 1062–71
Manolio TA, Furberg CD, Shemanski L, et al. Associations of postmenopausal estrogen use with cardiovascular disease and its risk factors in older women. Circulation 1993; 88 Pt 1: 2163–71
Ross RK, Paganini-Hill A, Mack TM, et al. Cardiovascular benefits of estrogen replacement therapy. Am J Obstet Gynecol 1989; 160: 1301–6
Woolley CS, Weiland NG, McEwen BS, et al. Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor mediated synaptic input: correlation with dendritic spine density. J Neurosci 1997; 17: 1848–59
Barraclough DJ, Brown MW, Ingram CD. 17-beta-oestradiol effects on long-term potentiation in the CA1 region of the hippocampus of the rat [abstract]. Brain Res Assoc Abstracts 1995; 12: 80
Gu Q, Moss RL. 17 Beta-estradiol potentiates kainate-induced currents via activation of the cAMP cascade. J Neurosci 1996; 16: 3620–9
Gibbs RB, Wu D, Hersh LB, et al. Effects of estrogen replacement on the relative levels of choline acetyltransferase, trkA, and nerve growth factor messenger RNAs in the basal forebrain and hippocampal formation of adult rats. Exp Neurol 1994; 129: 70–80
McMillan PJ, Singer CA, Dorsa DM. The effects of ovariectomy and estrogen replacement on trkA and choline acetyltransferase mRNA expression in the basal forebrain of the adult female Sprague-Dawley rat. J Neurosci 1996; 16(5): 1860–5
Simpkins JW, Singh M, Bishop J. The potential role for estrogen replacement therapy in the treatment of the cognitive decline and neurodegeneration associated with Alzheimer’s disease. Neurobiol Aging 1994; 15Suppl. 1: S195–7
Singh M, Meyer EM, Millard WJ, et al. Ovarian steroid deprivation results in a reversible learning impairment and compromised cholinergic function in female Sprague-Dawley rats. Brain Res 1994; 644: 305–12
O’Neal MF, Means LW, Poole MC, et al. Estrogen affects performance of ovariectomized rats in a two-choice water-escape working memory task. Psychoneuroendocrinology 1996; 21(1): 51–65
Hefti F, Schneider LS. Rationale for the planned clinical trials with nerve growth factor in Alzheimer’s disease. Psychiatr Dev 1989; 4: 297–315
Honjo H, Tamura T, Matsumoto Y, et al. Estrogen as a growth factor to central nervous cells. J Steroid Biochem 1992; 41: 633–5
Kendall DA, Stancel GM, Enna SJ. Imipramine: effect of ovarian steroids on modifications in serotonin receptor binding. Science 1981; 211: 1183–5
Fink G. Estrogen and mental state. Nature 1996; 383: 306–8
Paul SM, Axelrod J, Saavedra JM, et al. Estrogen-induced efflux of endogenous catecholamines from the hypothalamus in vitro. Brain Res 1979; 178: 499–505
Becker JB, Snyder PJ, Miller MM, et al. The influence of estrous cycle and intrastriatal estradiol on sensorimotor performance in the female rat. Pharmacol Biochem Behav 1987; 27: 53–9
McDermott JL, Kreutzberg JD, Liu B, et al. Effects of estrogen treatment on sensorimotor task performance and brain dopamine concentrations in gonadectomized male and female CD-1 mice. Horm Behav 1994; 28: 16–28
Roy EJ, Buyer DR, Lucari VA. Estradiol in the striatum: effects on behavior and dopamine receptors but no evidence for membrane steroid receptors. Brain Res Bull 1990; 25: 221–7
Joseph JA, Kochman K, Roth GS. Reduction of motor behavioral deficits in senescence via chronic prolactin or estrogen administration: time course and putative mechanisms of action. Brain Res 1989; 550: 195–202
Bishop J, Simpkins JW. Role of estrogens in peripheral and cerebral glucose utilization. Rev Neurosci 1992; 3(2): 121–37
Namba H, Sokoloff L. Acute administration of high doses of estrogen increases glucose utilization throughout the brain. Brain Res 1984; 291: 391–4
Bishop J, Simpkins JW. Estradiol enhances brain glucose transport and utilization in ovariectomized rats. Brain Res Bull 1993; 36: 315–20
Josefsson E, Tarkowski A, Carlsten H. Anti-inflammatory properties of estrogen. Cell Immunol 1992; 142: 67–78
Screpanti I, Santoni A, Gulino A, et al. Estrogen and anti-estrogen modulation of the levels of mouse natural killer activity and large granular lymphocytes. Cell Immunol 1987; 106: 191–202
Aisen PS, Davis KL. Inflammatory mechanisms in Alzheimer’s disease: implications for therapy. Am J Psychiatry 1994; 151: 1105–13
Finch CE, Marchalonis JJ. Evolutionary perspectives on amyloid and inflammatory features of Alzheimer disease. Neurobiol Aging 1996; 17: 809–16
Sherwin BB, Gelfand MM. A prospective one-year study of estrogen and progestin in postmenopausal women: effects on clinical symptoms and lipoprotein lipids. Obstet Gynecol 1989; 73: 759–66
Walsh BW, Schiff I, Rosner B, et al. Effects of postmenopausal estrogen replacement on the concentrations and metabolism of plasma lipoproteins. N Engl J Med 1991; 325: 1196–204
Shimano H, Murase T, Ishibashi S, et al. Plasma apolipoproteins in patients with multi-infarct dementia. Atherosclerosis 1989; 79: 257–60
Roses AD, Strittmatter WJ, Pericak-Vance MA, et al. Clinical application of apolipoprotein E genotyping to Alzheimer disease. Lancet 1994; 343: 1364–5
Poirier J. Apolipoprotein E in animal models of CNS injury and in Alzheimer’s disease. Trend Neurosci 1994; 17: 525–30
Stone DJ, Rozovsky I, Morgan TE, et al. Astrocytes and microglia respond to estrogen with increased apoE mRNA in vivo and in vitro. Exp Neurol 1997; 143: 313–8
Bertrand P, Poirier J, Oda T, et al. Association of apolipoprotein F genotype with brain levels of apolipoprotein E and apolipoprotein J (clusterin) in Alzheimer disease. Mol Brain Res 1995; 33: 174–8
Oda T, Wals P, Osterburg HH, et al. Clusterin (apoJ) alters the aggregation of amyloidβ-peptide (Aβ1-42) and forms slowly sedimenting Aβ complexes that cause oxidative stress. Exp Neurol 1995; 136: 22–31
Chao M, Spencer RL, Frankfurt M, et al. The effects of aging and hormonal manipulation on amyloid precursor protein APP695 mRNA expression in the rat hippocampus. J Neuroendocrinol 194; 6: 517–21
Katzman R, Kawas C. The epidemiology of dementia and Alzheimer disease. In: Terry RD, Katzman R, Bick KL, editors. Alzheimer disease. Raven Press: New York, 1994: 105–22
Rocca WA, Hofman A, Brayne C, et al. Frequency and distribution of Alzheimer’s disease in Europe: a collaborative study of 1980–1990 prevalence findings. The EURODEM Prevalence Research Group. nn Neurol 1991; 30(3): 381–90
Heyman A, Wilkinson WE, Stafford JA, et al. Alzheimer’s disease: a study of epidemiological aspects. Ann Neurol 1984; 15: 335–41
Amaducci LA, Fratiglioni L, Rocca WA, et al. Risk factors in clinically diagnosed Alzheimer’s disease: a case-control study of an Italian population. Neurology 1986; 36: 922–31
Broe GA, Henderson AS, Creasey H, et al. A case-control study of Alzheimer’s disease in Australia. Neurology 1990; 40: 1698–707
Graves AB, White E, Koepsell TD, et al. A case-control study of Alzheimer’s disease. Ann Neurol 1990; 28: 766–74
Barrett-Connor E, Kritz-Silverstein D. Estrogen replacement therapy and cognitive function in older women. JAMA 1993; 269: 2637–41
Henderson VW, Paganini-Hill A, Emanual CK, et al. Estrogen replacement therapy in older women: comparisons between Alzheimer’s disease and nondemented control subjects. Arch Neurol 1994; 51: 896–900
Brenner DE, Kukull WA, Stergachis A, et al. Postmenopausal estrogen replacement therapy and the risk of Alzheimer’s disease: a population-based case-control study. Am J Epidemiol 1994; 140: 262–7
Mortel KF, Meyer JS. Lack of postmenopausal estrogen replacement therapy and the risk of dementia. J Neuropsychiatr Clin Neurosci 1995; 7: 334–7
van Duijn CM, Meijer H, Witteman JCM, et al. Estrogen, apolipoprotein E and the risk of Alzheimer’s disease. Neurobiol Aging 1996; 17Suppl. 4S: S79–80
Lerner AJ, Koss E, Debanne SM, et al. Interactions of smoking history with estrogen replacement therapy as protective factors for Alzheimer’s disease. Lancet 1997; 349: 403–4
Paganini-Hill A, Henderson VW. Estrogen deficiency and risk of Alzheimer’s disease in women. Am J Epidemiol 1994; 140: 256–61
Tang MX, Jacobs D, Stern Y, et al. Effect of oestrogen during menopause on risk and age at onset of Alzheimer’s disease. Lancet 1996; 348: 429–32
Kawas C, Resnick S, Morrison A, et al. A prospective study of estrogen replacement therapy and the risk of developing Alzheimer’s disease: the Baltimore Longitudinal Study of Aging. Neurology. In press
Phillips SM, Sherwin BB. Effects of estrogen on memory function in surgically menopausal women. Psychoneuroendocrinology 1992; 17: 485–95
Sherwin BB. Estrogen and/or androgen replacement therapy and cognitive functioning in surgically menopausal women. Psychoneuroendocrinology 1988; 13: 345–57
Kampen DL, Sherwin BB. Estrogen use and verbal memory in healthy postmenopausal women. Obstet Gynecol 1994; 83: 979–83
Caldwell BM, Watson RI. An evaluation of psychological effects of sex hormone administration in aged women: I. Results of therapy after six months. J Gerontol 1952; 7: 228–44
Kantor HI, Michael CM, Shore H, et al. Administration of estrogens to older women: a psychometric evaluation. Am J Obstet Gynecol 1968; 101: 658–61
Fillit H, Weinreb H, Cholst I, et al. Observations in a preliminary open trial of estradiol therapy for senile dementia-Alzheimer’s type. Psychoneuroendocrinology 1986; 11: 337–45
Weiss BL. Failure of nalmefene and estrogen to improve memory in Alzheimer’s disease. Am J Psychiatry 1987; 144: 386–7
Honjo H, Ogino Y, Naitoh K, et al. In vivo effects by estrone sulfate on the central nervous system-senile dementia (Alzheimer’s type). J Steroid Biochem 1989; 34: 521–5
Ohkura T, Isse K, Akazawa K, et al. Evaluation of estrogen treatment in female patients with dementia of the Alzheimer type. Endocr J 1994; 41: 361–71
Ohkura T, Isse K, Akazawa K, et al. Low-dose estrogen replacement therapy for Alzheimer’s disease in women. Menopause 1994; 1: 125–30
Ohkura T, Isse K, Akazawa K, et al. Long-term estrogen replacement therapy in female patients with dementia of the Alzheimer type: 7 case reports. Dementia 1995; 6(2): 99–107
Honjo H, Ogino Y, Tanaka K, et al. An effect of conjugated estrogen to cognitive impairment in women with senile dementia-Alzheimer’s type: a placebo-controlled double blind study. J Jpn Menopause Soc 1993; 1(2): 167–71
Asthana S, Craft S, Baker D, et al. Transdermal estrogen improves memory in women with Alzheimer’s disease [abstract]. Soc Neurosci 1996; 22: 200
Schneider LS, Farlow MR, Henderson VW, et al. Effects of estrogen replacement therapy on response to tacrine in patients with Alzheimer’s disease. Neurology 1996; 46: 1580–4
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schneider, L.S., Finch, C.E. Can Estrogens Prevent Neurodegeneration?. Drugs & Aging 11, 87–95 (1997). https://doi.org/10.2165/00002512-199711020-00001
Published:
Issue Date:
DOI: https://doi.org/10.2165/00002512-199711020-00001