Abstract
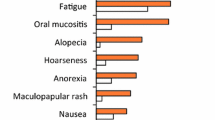
Sunitinib is a novel, oral, multi-targeted tyrosine kinase inhibitor with antiproliferative effects against cancer cells and antiangiogenic properties. Sunitinib was recently approved for the first-line treatment of patients with advanced renal cell carcinoma (RCC) and for the treatment of patients with gastrointestinal stromal tumours (GIST) after disease progression or intolerance to imatinib therapy. The main purpose of this benefit-risk assessment is to review data on sunitinib efficacy along with its toxicity in patients with GIST and RCC. Sunitinib demonstrates a high level of efficacy with acceptable tolerability using either the 50 mg daily oral dosing for 4 weeks every 6 weeks or a continuous daily administration schedule at a lower dose. Hypertension and asthenia appear to be the most common adverse effects with sunitinib. Diarrhoea, anorexia, disgeusia, stomatitis and skin toxicity are other clinically relevant toxicities. Fatigue may, at least in part, be related to the development of hypothyroidism during sunitinib therapy. Skin toxicity consists of bullous lesion in the soles and palms that may require treatment discontinuation for a few days and/or dose reduction. Thyroid hormone levels should be monitored during treatment with sunitinib, with the occurrence of clinical signs of hypothyroidism needing treatment with levothyroxine sodium. Hypertension usually requires standard antihypertensive therapy and treatment discontinuation is less frequently necessary. Mild neutropenia and thrombocytopenia usually require no intervention. A decrease in left ventricular ejection fraction is a rare but potentially life-threatening complication. Although usually well tolerated, sunitinib needs to be administered cautiously with medical follow-up in patients with cancer to prevent, avoid and treat adverse effects in order to improve patient compliance. Its established antitumor activity requires attempting to maintain the highest tolerable dose in individual patients. Current oral formulations allow physicians to modulate dosages (between 25 and 50 mg/day) and/or schedules (4 weeks on, 2 weeks off or continuous administration) to optimize the benefit-risk profile of sunitinib in individual patients.






Similar content being viewed by others
References
Faivre S, Djelloul S, Raymond E. New paradigms in anticancer therapy: targeting multiple signalling pathways with kinase inhibitors. Semin Oncol 2006; 33: 407–20
Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon a in metastatic renal-cell carcinoma. N Engl J Med 2007; 356: 115–24
Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomized controlled trial. Lancet 2006; 368: 1329–38
Goodman VL, Rock EP, Dagher R, et al. Approval summary: sunitinib for the treatment of imatinib refractory or intolerant gastrointestinal stromal tumors and advanced renal cell carcinoma. Clin Cancer Res 2007 Mar 1; 13(5): 1367–73
Faivre S, Demetri G, Sargent W, et al. Molecular basis for sunitinib efficacy and future clinical development. Nat Rev Drug Discov 2007 Sep; 6(9): 734–45
Duensing A, Heinrich MC, Fletcher CD, et al. Biology of gastrointestinal stromal tumors: KIT mutations and beyond. Cancer Invest 2004; 22: 106–16
Naoe T, Kiyoi H. Normal and oncogenic FLT3. Cell Mol Life Sci 2004; 61: 2932–8
Ichihara M, Murakumo Y, Takahashi M. RET and neuroendocrine tumors. Cancer Lett 2004; 204: 197–211
Sapi E. The role of CSF-1 in normal physiology of mammary gland and breast cancer: an update. Exp Biol Med 2004; 229: 1–11
Barattè S, Sarati S, Frigerio E, et al. Quantitation of SU11248, an oral multi-target tyrosine kinase inhibitor, and its metabolite in monkey tissues by liquid chromatograph with tandem mass spectrometry following semiautomated liquid-liquid extraction. J Chromatogr A 2004 Jan 23; 1024(1–2): 87–94
Bello CL, Sherman L, Zhou J, et al. Effect of food on the pharmacokinetics of sunitinib malate (SU11248), a multitargeted receptor tyrosine kinase inhibitor: results from a phase I study in healthy subjects. Anticancer Drugs 2006; 17: 353–8
De Mulder PH, Roigas J, Gillessen S, et al. A phase II study of sunitinib administered in a continuous daily regimen in patients with cytokine refractory metastatic renal cell carcinoma (mRCC). American Society of Clinical Oncology 42nd Annual Meeting; 2006 Jun 2–6; Atlanta (GA)
George S, Casali PG, Blay J, et al. Phase II study of sunitinib administered in a continuous daily dosing regimen in patients (pts) with advanced GIST. American Society of Clinical Oncology 42nd Annual Meeting; 2006 Jun 2–6; Atlanta (GA)
Blay JY, George S, Casali PG, et al. Clinical benefit of continuous daily dosing of sunitinib in patients (pts) with advanced gastrointestinal stromal tumor (GIST) [abstract]. Ann Oncol 2006; 17 Suppl. 9: 163
Escudier B, Roigas J, Gillessen S, et al. Continuous daily administration of sunitinib malate (SU 11248): a phase II study in patients (pts) with cytokine-refractory metastatic renal cell carcinoma (mRCC) [abstract]. Ann Oncol 2006; 17 Suppl. 9: 144
Mendel DB, Laird AD, Xin X, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and plateletderived growth factor receptors: determination of a pharmacokinetic/ pharmacodynamic relationship. Clin Cancer Res 2003; 9: 327–37
Abrams TJ, Lee JB, Murray LJ, et al. SU1 1248 inhibits KIT and platelet derived growth factor receptor b in preclinical models of human small cell lung cancer. Mol Cancer Ther 2003; 2: 471–8
Abrams TJ, Murray LJ, Pesenti E, et al. Preclinical evaluation of the tyrosine kinase inhibitor SU1 1248 as a single agent and in combination with “standard of care” therapeutic agents for the treatment of breast cancer. Mol Cancer Ther 2003; 2: 1011–21
Murray LJ, Abrams TJ, Long KR, et al. SU11248 inhibits tumor growth and CSF-1R-dependent osteolysis in an experimental breast cancer bone metastasis model. Clin Exp Metastasis 2003; 20: 757–66
Faivre S, Delbaldo C, Vera K, et al. Safety, pharmacokinetic, and antitumor activity of SU1 1248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol 2006; 24: 25–35
Motzer RJ, Hoosen S, Bello CL, et al. Sunitinib malate for the treatment of solid tumors: a review of current clinical data. Expert Opin Investig Drugs 2006; 15: 553–61
Lam JS, Leppert JT, Belldegrun AS, et al. Novel approaches in the therapy of metastatic renal cell carcinoma. World J Urol 2005; 23: 202–12
Motzer RJ, Bacik J, Schwartz LH, et al. Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol 2004; 22: 454–63
Rohrmann K, Staehler M, Haseke N, et al. Immunotherapy in metastatic renal cell carcinoma. World J Urol 2005; 23: 196–201
Motzer RJ, Bander NH, Nanus DM, et al. Renal-cell carcinoma. N Engl J Med 1996; 335: 865–75
Patel PH, Chaganti RSK, Motzer RJ. Targeted therapy for metastatic renal cell carcinoma. Br J Cancer 2006; 94: 614–9
Kamura T, Sato S, Iwai K, et al. Activation of HIF1alpha ubiquitination by a reconstituted von Hippel-Lindau (VHL) tumor suppressor complex. Proc Natl Acad Sci U S A 2000; 97: 10430–5 733
Wang GL, Semenza GL. General involvement of hypoxiainducible factor 1 in transcriptional response to hypoxia. Proc Natl Acad Sci U S A 1993; 90: 4304–8
Bardos JL, Aschcroft M. Hypoxia-inducible factor-1 and oncogenic signalling. Bioessays 2004; 26: 262–9
Motzer RJ, Michaelson MD, Redman BG, et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol 2006; 24: 16–24
Motzer RJ, Rini BI, Bukowski RM, et al. Sunitinib in patients with metastatic renal cell carcinoma. JAMA 2006; 295: 2516–24
Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet 2007; 370: 2103–11
Heng DY, Chi KN, Murray N, et al. A population-based study evaluating the impact of sunitinib on overall survival in the treatment of patients with metastatic renal cell cancer. Cancer 2009 Feb 15; 115(4): 776–83
Margulis V, Matin SF, Tannir N, et al. Surgical morbidity associated with administration of targeted molecular therapies before cytoreductive nephrectomy or resection of locally recurrent renal cell carcinoma. Urol Oncol 2009 Jan–Feb; 27(1): 108–9
Heinrich MC, Corless MC, Demetri GD, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol 2003; 21: 4342–9
Corless CL, Fletcher JA, Heinrich MC. Biology of gastrointestinal stromal tumors. J Clin Oncol 2004; 22: 3813–25
Corless CL, Schroeder A, Griffith D, et al. PDGFRA mutations in gastrointestinal stromal tumors: frequency, spectrum and in vitro sensitivity to imatinib. J Clin Oncol 2005 Aug 10; 23(23): 5357–64
Blanke CD, Corless CL. State-of-the art therapy for gastrointestinal stromal tumors. Cancer Invest 2005; 23: 274–80
Demetri GD, Von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 2002; 347: 472–80
Van Glabbeke M, Verweij J, Casali PG, et al. Initial and late resistance to imatinib in advanced gastrointestinal stromal tumors are predicted by different prognostic factors: a European Organisation for Research and Treatment of Cancer-Italian Sarcoma Group-Australasian Gastrointestinal Trials Group study. J Clin Oncol 2005; 23: 5795–804
Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumors with high-dose imatinib: randomised trial. Lancet 2004; 364: 1127–34
Maki RG, Fletcher JA, Heinrich MC, et al. SU 11248 in patients with imatinib-resistant GIST: results from a continuation trial. American Society of Clinical Oncology 41st Annual Meeting; 2005 May 13–17; Orlando (FL)
Demetri GD, Huang X, Garrett CR, et al. Novel statistical analysis of long-term survival to account for crossover in a phase III trial of sunitinib (SU) vs. placebo (PL) in advanced GIST after imatinib (IM) failure [abstract]. J Clin Oncol 2008 May 20; 26 Suppl.: 10524
Reichardt P, Kang Y, Ruka W, et al. Detailed analysis of survival and safety with sunitinib (SU) in a worldwide treatment-use trial of patients with advanced GIST [abstract]. J Clin Oncol 2008 May 20; 26 Suppl.: 10548
Blackstein M, Huang X, Demetri GD, et al. Investigation of soluble kit as a potential surrogate marker for ttp in sunitinib-treated patients with GIST [abstract]. Ann Oncol 2007; 18 (Suppl. 7): VII16
Heinrich MC, Maki RG, Corless CL, et al. Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumor. J Clin Oncol 2008 Nov 20; 26(33): 5352–9
SUTENT product monograph [online]. Available from URL: http://www.pfizer.ca/english/our%20products/prescription%20pharmaceuticals/default.asp?s=1&id=32&doc=enmonograph [Accessed 2009 Jun 16]
Navari RM, Koeller JM. Electrocardiographic and cardiovascular effects of the 5-hydroxytryptamine3 receptor antagonists. Ann Pharmacother 2003; 37: 1276–86
Rini BI, Tamaskar I, Shaheen P, et al. Hypothyroidism in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst 2007; 99: 81–3
Desai J, Yassa L, Marqusee E, et al. Hypothyroidism after sunitinib treatment for patients with gastrointestinal stromal tumors. Ann Intern Med 2006; 145: 660–4
Shaheen PE, Tamaskar IR, Salas RN, et al. Thyroid function tests (TFTs) abnormalities in patients (pts) with metastatic renal cell carcinoma (mRCC) treated with sunitinib [abstract]. Proc Am Soc Clin Oncol 2006; 24: 4605
Schoeffski P, Wolter P, Himpe U, et al. Sunitinib-related thyroid dysfunction: a single-center retrospective and prospective evaluation [abstract]. Proc Am Soc Clin Oncol 2006; 24: 3092
Grossmann M, Premaratne E, Desai J, et al. Thyrotoxicosis during sunitinib treatment for renal cell carcinoma. Clin Endocrinol (Oxf) 2008 Oct; 69(4): 669–72
Sica DA. Angiogenesis inhibitors and hypertension: an emerging issue. J Clin Oncol 2006; 24: 1329–31
Willett CG, Boucher Y, Di Tomaso E, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med 2004; 10: 145–7
Yang JC, Haworth L, Sherry RM, et al. A randomized trial of bevacizumab, an antivascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med 2003; 349: 427–34
Ahmad T, Eisen T. Kinase inhibition with BAY 43-9006 in renal cell carcinoma. Clin Cancer Res 2004; 10: 6388–92S
Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 2007; 356: 125–34
Veronese ML, Mosenkis A, Flaherty KT, et al. Mechanisms of hypertension associated with BAY 43-9006. J Clin Oncol 2006; 24: 1363–9
Rixe O, Billemont B, Izzedine H. Hypertension as a predictive factor of sunitinib activity [abstract]. Ann Oncol 2007 Jun; 18(6): 1117
Dougall HT, McLay J. A comparative review of the adverse effects of calcium antagonists. Drug Saf 1996; 15: 91–106 734
Cross BW, Kirby MG, Miller S, et al. A multicentre study of the safety and efficacy of amlodipine in mild to moderate hypertension. Br J Clin Pract 1993; 47: 237–40
Pedrinelli R, Dell’Omo G, Melillo E, et al. Amlodipine, enalapril, and dependent leg edema in essential hypertension. Hypertension 2000; 35: 621–5
Ruka W, Rutkowski P, Nowecki Z, et al. Emergency surgery due to complications during molecular targeted therapy in advanced gastrointestinal stromal tumors (GIST). Society of Surgical Oncology 61st Annual Cancer Symposium, Chicago (IL), 13–16 March 2008 [abstract]. Ann Surg Oncol 2008; 15 suppl. 2: 82
Telli ML, Witteles RM, Fisher GA, et al. Cardiotoxicity associated with the cancer therapeutic agent sunitinib malate. Ann Oncol 2008; 19(9): 1613–8
Khakoo AY, Kassiotis CM, Tannir N, et al. Heart failure associated with sunitinib malate: a multitargeted receptor tyrosine kinase inhibitor. Cancer 2008 Jun 1; 112(11): 2500–8
Chu TF, Rupnick MA, Kerkela R, et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet 2007 Dec 15; 370(9604): 2011–9
Wolter P, Stefan C, Decallonne B, et al. The clinical implications of sunitinib-induced hypothyroidism: a prospective evaluation. Br J Cancer 2008 Aug 5; 99(3): 448–54
Robert C, Soria JC, Spatz A, et al. Cutaneous side-effects of kinase inhibitors and blocking antibodies. Lancet Oncol 2005; 6: 491–500
Lacouture ME, Reilly LM, Gerami P, et al. Hand foot skin reaction in cancer patients treated with the multikinase inhibitors sorafenib and sunitinib. Ann Oncol 2008 Jun 10; 19(11): 1955–61
Tsai KY, Yang CH, Kuo TT, et al. Hand-foot syndrome and seborrheic dermatitis-like rash induced by sunitinib in a patient with advanced renal cell carcinoma. J Clin Oncol 2006; 24: 5786–8
Alexandrescu DT, Vaillant JG, Dasanu CA. Effect of treatment with a colloidal oatmeal lotion on the acneform eruption induced by epidermal growth factor receptor and multiple tyrosine-kinase inhibitors. Clin Exp Dermatol 2007; 32: 71–4
Botchkareva NV, Khlgatian M, Longley BJ, et al. SCF/KIT signaling is required for cyclic regeneration of the hair pigmentation unit. FASEB J 2001; 15: 645–58
Hemesath TJ, Price ER, Takemoto C, et al. MAP kinase links the transcription factor microphthalmia to KIT signalling in melanocytes. Nature 1998; 391: 298–301
Moss KG, Toner GC, Cherrington JM, et al. Hair depigmentation is a biological readout for pharmacological inhibition of KIT in mice and humans. J Pharmacol Exp Ther 2003; 307: 476–80
George S, Blay JY, Casali PG, et al. Continuous daily dosing (CDD) of sunitinib (SU) in pts with advanced GIST: updated efficacy, safety, PK and pharmacodynamic analysis [abstract]. J Clin Oncol 2008 May 20; 26: 10554
Houk BE, Bello CL, Michaelson MD, et al. Exposureresponse of sunitinib in metastatic renal cell carcinoma (mRCC): a population pharmacokinetic/pharmacodynamic (PKPD) approach [abstract]. J Clin Oncol 2007; 25: 5027
van der Veldt AA, Boven E, Helgason HH, et al. Predictive factors for severe toxicity of sunitinib in unselected patients with advanced renal cell cancer. Br J Cancer 2008; 99: 259–65
Shukla S, Robey RW, Bates SE, et al. Sunitinib (Sutent®, SU 11248), a small-molecule receptor tyrosine kinase inhibitor, blocks function of the ABC transporters, P-glycoprotein (ABCB1) and ABCG2. Drug Metab Dispos 2009 Feb; 37(2): 359–65
Acknowledgements
No funding was received for the preparation of this benefitrisk assessment. Eric Raymond has been a consultant to and received honoraria from Pfizer Inc. Sandrine Faivre has received honoraria from Pfizer Inc. None of the other authors have any conflicts of interest relevant to the content of this benefit-risk assessment.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Theou-Anton, N., Faivre, S., Dreyer, C. et al. Benefit-Risk Assessment of Sunitinib in Gastrointestinal Stromal Tumours and Renal Cancer. Drug-Safety 32, 717–734 (2009). https://doi.org/10.2165/00002018-200932090-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00002018-200932090-00003