Abstract
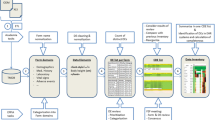
Multi-site clinical protocols and clinical research networks require tools to manage and monitor adverse events (AEs). To be successful, these tools must be designed to comply with applicable regulatory requirements, reflect current data standards, international directives and advances in pharmacovigilance, and be convenient and adaptable to multiple needs. We describe an Adverse Event Data Management System (AEDAMS) that is used across multiple study designs in the various clinical research networks and multi-site studies for which we provide data and technological support. Investigators enter AE data using a standardized and structured web-based data collection form. The automated AEDAMS forwards the AE information to individuals in designated roles (investigators, sponsors, Data Safety and Monitoring Boards) and manages subsequent communications in real time, as the entire reporting, review and notification is done by automatically generated emails. The system was designed to adhere to timelines and data requirements in compliance with Good Clinical Practice (International Conference on Harmonisation E6) reporting standards and US federal regulations, and can be configured to support AE management for many types of study designs and adhere to various domestic or international reporting requirements. This tool allows AEs to be collected in a standard way by multiple distributed users, facilitates accurate and timely AE reporting and reviews, and allows the centralized management of AEs. Our design justification and experience with the system are described.
Similar content being viewed by others
References
Friedman LM, Furberg CD, DeMets DL. Assessing and reporting adverse events. In: Fundamentals of clinical trials. Third ed. New York: Springer-Verlag; 1998: 170–184
Raman R, Thomas R. A centralized serious adverse event reporting and coding system for multi-center clinical trials: an academic research organization experience. 27th Annual Meeting of the Society for Clinical Trials; 2006 May 21-4, Orlando (FL)
Malloy J, Richesson RL, Krischer J. The adverse event management system for the Rare Disease Clinical Research Network. Inventory of Clinical Research Networks, National Leadership Forum; 2006 May 31-Jun 1; Washington, DC
Felten SJ, Mandrekar SJ, Tan AD, et al. A web-based system for monitoring phase I clinical trials: the Mayo Clinic experience. In: 27th Annual Meeting of the Society for Clinical Trials; 2006 May 21-4, Orlando
National Cancer Institute. Adverse Event Expedited Reporting System (AdEERS) [online]. Available from URL: http://ctep.cancer.gov/reporting/adeers.html [Accessed 2008 Aug 8]
Landis JR, Curley RM, Dwyer W, et al. Emerging partnership between NIH roadmap re-engineering of clinical research networks and oracle corporation’s adverse events reporting system (AERS®). Inventory of Clinical Research Networks, National Leadership Forum; 2006 May 31-Jun 1; Washington, DC
National Institutes of Health Press Release. NIH and FDA launch new human gene transfer research data system. GeM-CRIS will facilitate faster reporting of adverse events in human gene transfer trials [online]. Available from URL: http://www.nih.gov/news/pr/mar2004/od-26.htm [Accessed 2008 Aug 12]
Holzmueller CG, Pronovost PJ, Dickman F, et al. Creating the web-based intensive care unit safety reporting system. J Am Med Inform Assoc 2005; 12: 130–9
Mekhjian HS, Bentley TD, Ahmad A, et al. Development of a web-based event reporting system in an academic environment. J Am Med Inform Assoc 2004; 11: 11–8
Takeda H, Matsumuraa Y, Nakajimab K, et al. Health care quality management by means of an incident report system and an electronic patient record system. Int J Med Inform 2003; 69(2-3): 285–93
Kivlahan C, Sangster W, Nelson K, et al. Developing a comprehensive electronic adverse event reporting system in an academic health center. Jt Comm J Qual Improv 2002; 28(11): 583–94
Hayney MS. Vaccine-adverse event reporting system: an essential tool for monitoring vaccine safety. J Am Pharm Assoc (2003) 2006; 46(2): 298–9
Chen RT, Rastogi SC, Mullen JR, et al. The vaccine adverse event reporting system (VAERS). Vaccine 1994; 12(6): 542–50
Vaccine adverse event reporting system: United States. MMWR Morb Mortal Wkly Rep 1990; 39(41): 730–3
Varricchio F. The vaccine adverse event reporting system. J Toxicol Clin Toxicol 1998; 36(7): 765–8
Centers for Disease Control and Prevention (CDC). Assessing the National Electronic Injury Surveillance System-Cooperative Adverse Drug Event Surveillance project: six sites, United States, Jan 1-Jun 15, 2004. MMWR Morb Mortal Wkly Rep 2005 Apr 22; 54(15): 380–3
GeMCRIS. Genetic Modification Clinical Research Information System version 4.0 [online]. Available from URL: http://www.gemcris.od.niuh.gov [Accessed 2008 Jul 2]
Silverman DI, Cirullo L, fMartinis NA, et al. Systematic identification and classification of adverse events in human research. Contemp Clin Trials 2006; 27(3): 295–303
Temple University. Temple IRB serious adverse event reporting [online]. Available from URL: http://www.temple.edu/ovpr/oct/oct_irb_sae_rprt.html [Accessed 2008 Jan 28]
Weiner MG, Livshits A, Carozzoni C, et al. Information systems developments to detect and analyze chemotherapy-associated adverse drug events [abstract]. American Medical Informatics Annual Symposium; 2002 Nov 9–13, San Antonio
Murff HJ, Fiskio JM, Bates DW. Electronically screening discharge summaries for adverse medical events. American Medical Informatics Annual Symposium; 2001 Nov 3–7, Washington, DC
Einbinder JS, Scully K. Using a clinical data repository to estimate the frequency and costs of adverse drug events. American Medical Informatics Annual Symposium; 2001 Nov 3–7, Washington, DC
Curioso WH, Karras BT, Campos PE, et al. Design and implementation of cell-PREVEN: a real-time surveillance system for adverse events using cell phones in Peru. American Medical Informatics Association Annual Symposium; 2005 Oct 22-26, 180
Forster AJ, Andrade J, van Walraven C. Validation of a discharge summary term search method to detect adverse events. J Am Med Inform Assoc 2005; 12(2): 200–6
Melton GB, George Hripcsak G. Automated detection of adverse events using natural language processing of discharge summaries. J Am Med Inform Assoc 2005; 12(4): 448–57
Hazlehurst B, Frost HR, Sittig DF, et al. Medi Class: a system for detecting and classifying encounter-based clinical events in any electronic medical record. J Am Med Inform Assoc 2005; 12(5): 517–29
Friedlin J, McDonald CJ. A natural language processing system to extract and code concepts relating to congestive heart failure from chest radiology reports. In: Bates DW, editor. American Medical Informatics Association Annual Symposium; 2006 Nov 11-15, 273
Penz JFE, Wilcox AE, Hurdle JF. Automated identification of adverse events related to central venous catheters. J Biomed Inform 2007; 40(2): 174–82
Welsh CH, Pedot R, Anderson RJ. Use of morning report to enhance adverse event detection. J Gen Intern Med 1996; 11(8): 454–60
Bates DW, Evans RS, Murff H, et al. Policy and the future of adverse event detection using information technology. J Am Med Inform Assoc 2003; 10(2): 226–8
McDaniel AM. Automated adverse event detection. Clin Nurse Spec 2004; 18(6): 273–4
Handler SM, Altman RL, Perera S, et al. A systematic review of the performance characteristics of clinical event monitor signals used to detect adverse drug events in the hospital setting. J Am Med Inform Assoc 2007; 14(4): 451–8
The Environmental Determinants of Diabetes in the Young (TEDDY) Study Project Website [online]. Available from URL: http://teddy.epi.wf.edu [Accessed 2008 Aug 8]
TRIGR Project Website [online]. Available from URL: http://trigr.epi.usp.edu [Accessed 2008 Aug 8]
Hagopian WA, Lernmark A, Rewers MJ. TEDDY — The Environmental Determinants of Diabetes in the Young: an observational clinical trial [online]. Available from URL: http://www.nnalsnyas.org/cgi/content/abstract/1079/1/320 [Accessed 2008 Aug 12]
Tiittanen M, Paronen J, Savilahti E, et al. Dietary insulin as an immunogen and tolerogen. Pediatr Allergy Immunol 2006; 17(7): 538–43
TRIGR study group. Study design of the Trial to Reduce IDDM in the Genetically at Risk (TRIGR). Pediatr Diabetes 2007; 8(3): 117–37
National Institutes for Health. NIH News. National Centre for Research Resources. NIH establishes rare diseases clinical research network [online]. Available from URL: http://www.nih.gov/news/pr/nov2003/ncrr-03.htm [Accessed 2008 Aug 8]
Hampton T. Rare disease research gets boost. JAMA 2006; 295: 2836–8
International Conference of Harmonization. Harmonised tripartite guideline. Guideline for good clinical practice E6 (R1) [online]. Available from URL: http://www.ich.org/LOB/med-ia/MEDIA482.pdf [Accessed 2007 Sep 15]
International Conference of Harmonization. Draft Consensus Guideline. Data elements for transmission of individual case safety reports E2B(R3) [online]. Available from URL: http://www.ich.org/LOB/media/MEDIA632.pdf [Accessed 2007 Sep 15]
International Conference of Harmonization. Harmonised tripartite guideline. Clinical safety data management: definitions and standards for expedited reporting E2A [online]. Available from URL: http://www.ich.org/LOB/media/MEDIA436.pdf[Accessed 2007 Sep 15]
US Food and Drug Administration. Guidance for industry. Part 11: electronic records; electronic signatures: scope and application [online]. Available from URL: http://www.fda.gov/CBER/gdlns/compclintrial.htm [Accessed 2007 Sep 15]
Brandt CA, Argraves S, Money R, et al. Informatics tools to improve clinical research study implementation. Contemp Clin Trials 2005; 27(2): 112–22
Welker JE. Implementation of electronic data capture systems: barriers and solutions. Contemp Clinical Trials 2007; 28(3): 329–36
Lopez-Carrero C, Arriaza E, Bolanos E, et al. Internet in clinical research based on a pilot experience. Contemp Clin Trials 2005; 26: 234–43
Richesson RL, Fung KW, Krischer JP. Heterogeneous but ’standard’ coding systems for adverse events: issues in achieving interoperability between apples and oranges. Contemp Clin Trials 2008; in press [online]. Available from URL: http://www.journals.elsevierhealth.com/periodicals/concli/article/51551-7144(08)00026-8/fulltext [Accessed 2008 Aug 19]
WHO. The safety of medicines in public health programmes: pharmacovigilance an essential tool. Geneva: WHO Press; 2006
U.S. Food and Drug Administration. Guidance for industry good pharmacovigilance practices and pharmacoepidemio-logic assessment [online]. Available from URL: http://www.f-da.gov/Cder/Guidance/6359OCC.htm [Accessed 2007 Sep 15]
Dreier G, Marx C, Schmoor C, et al. The 12th amendment to the German drug law. Chances and obstacles for investigator-initiated clinical trials [German]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2005; 48(4): 445–52
WHO. Management of safety information from clinical trials, report of CIOMS working group VI. Geneva: WHO; 2005
Schmier JK, Kane DW, Halpern MT. Practical applications of usability theory to electronic data collection for clinical trials. Contemp Clin Trials 2005; 26(3): 376–85
Levin R. Data standards for regulated clinical trials: FDA perspective [online]. Available from URL: http://www.cdisc.org/pdf/2004_06_14_cdisc.pdf [Accessed 2008 Aug 7]
Bodenreider O. The unified medical language system (UMLS): integrating biomedical terminology. Nucleic Acids Res 2004; 32 (Database issue): D267-70
National Cancer Institute. List of codes and values [online]. Available from URL: http://ctep.cancer.gov/guidelines/codes.html [Accessed 2008 Aug 7]
Segal ES, Valette C, Oster L, et al. Risk management strategies in the postmarketing period: safety experience with the US and European bosentan surveillance programmes. Drug Saf 2005; 28(11): 971–80
Pronovost PJ, Holzmueller CG, Young J, et al. Using incident reporting to improve patient safety: a conceptual model. J Patient Saf 2007; 3(1): 27–33 au58._Zerhouni EA. Keynote presentation. American Medical Informatics Association Annual Symposium; 2005 Oct 23, Washington, DC
Brandt CA, Cohen DB, Shifman MA, et al. Approaches and informatics tools to assist in the integration of similar clinical research questionnaires. Methods Inform Med 2004; 43: 156–62
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Richesson, R.L., Malloy, J.F., Paulus, K. et al. An Automated Standardized System for Managing Adverse Events in Clinical Research Networks. Drug-Safety 31, 807–822 (2008). https://doi.org/10.2165/00002018-200831100-00001
Published:
Issue Date:
DOI: https://doi.org/10.2165/00002018-200831100-00001