Abstract
Introduction: Monitoring was required for the introduction of non-chlorofluorocarbon (CFC) propellants in metered dose inhalers (MDIs) to ensure that there were no unexpected adverse events due to the new products. A postmarketing surveillance study has been conducted to evaluate the introduction of the MDI Seretide Evohaler™ (hydrofluoroalkane-134a inhaler containing salmeterol and fluticasone propionate).
Objectives: To summarise the modified prescription-event monitoring (PEM) study conducted to evaluate the introduction of Seretide Evohaler™ and discuss the relevance of this type of study towards pharmacovigilance risk-management planning.
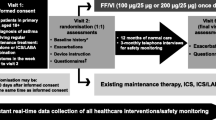
Methods: Modified PEM methodology was used to examine the introduction of Seretide Evohaler™ into general practice in England. Patients were identified from the first National Health Service prescriptions dispensed in England for Seretide Evohaler™. One postal questionnaire was sent to the prescribing doctor, requesting demographic information, severity of the indication, concomitant medication for this condition, smoking history, event data 3 months prior to and 3 months after the first prescription for Seretide Evohaler™ and also reason for stopping if it had been stopped. Pregnancies, deaths and selected events were followed up. Incidence density ratios were calculated to compare event rates 3 months prior to and 3 months after the introduction of Seretide Evohaler™. A matched cohort analysis examined oral corticosteroid use and hospital admissions between the pre-and post-exposure periods.
Results: The cohort comprised 13 464 patients prescribed Seretide Evohaler™, with a response rate of 62%. There was no significant difference in the length of courses of oral corticosteroid use when the pre-and post-exposure periods were compared. A matched cohort analysis showed there was no increase in the use of oral corticosteroids (relative risk [RR] 0.95; 95% CI 0.90, 0.99) or hospital admissions in the post-exposure period (RR 0.87; 95% CI 0.73, 1.04). When the number of patients with events were compared for the periods 3 months before and 3 months after exposure, fewer events were reported in the post-exposure period. There were 64 patients who experienced adverse events within an hour of using Seretide Evohaler™, including one report of paradoxical bronchospasm and one of myocardial infarction with fatal outcome that were both assessed as possibly related to treatment.
Discussion: The results of the study suggest that the introduction of Seretide Evohaler™ was generally well tolerated. The modified methodology has allowed a comparison of the event rates before and after the introduction of this CFC-free inhaler into general practice.






Similar content being viewed by others
Notes
1The use of trade names is for product identification purposes only and does not imply endorsement.
2Doses are stated as salmeterol/fluticasone propionate.
References
The Montreal protocol on substances that deplete the ozone layer. 2000 [online]. Available from URL: http://www.unep.org/ozone/Montreal-Protocol/Montreal-Protocol2000.shtml [Accessed 2006 Aug 23]
Ibiapina CC, Cruz AA, Camargos PA. Hydrofluoroalkane as a propellant for pressurized metered-dose inhalers: history, pulmonary deposition, pharmacokinetics, efficacy and safety [in Portuguese]. J Pediatr (Rio J) 2004; 80(6): 441–6
Leach CL. The CFC to HFA transition and its impact on pulmonary drug development. Respir Care 2005; 50(9): 1201–8
Gates BJ, Neumiller JJ. CFC Phaseout and Adances in Oral Inhalers. Advances in Pharmacy 2005; 3(2): 131–142, 155
Zeidler M, Corren J. Hydrofluoroalkane formulations of inhaled corticosteroids for the treatment of asthma. Treat Respir Med 2004; 3(1): 35–44
European Commission. European Community strategy for the phaseout of CFCs in metered dose inhalers, 1998 [online]. Available from URL: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:51998DC0603:EN:HTML [Accessed 2006 Aug 23]
European Agency for the Evaluation of Medicinal Products. Guideline for PMS studies for metered dose inhalers with new propellants. CPMP/180/95. 1995 [online]. Available from URL: http://www.emea.eu.int/pdfs/human/qwp/018095en.pdf [Accessed 2006 Sep 1]
International Conference on Harmonisation of Technical Requirements for Registration of Medicinal Products for Human Use. Pharmacovigilance planning E2E. 2004 [online]. Available from URL: http://www.ich.org [Accessed 2006 Aug 23]
Shakir SAW. Prescription-event monitoring. In: Strom BL, editor. Pharmacoepidemiology. 4th ed. Chichester, UK: John Wiley & Sons Ltd, 2005: 203–16
Allen & Hanburys. Seretide Accuhaler, summary of product characteristics. 2005
Allen & Hanburys. Flixotide Evohaler, summary of product characteristics. 2000
Allen & Hanburys. Serevent Evohaler, summary of product characteristics. 2006
Allen & Hanburys. Seretide Evohaler, summary of product characteristics. 2000
Reynolds NA, Lyseng-Williamson KA, Wiseman LR. Inhaled salmeterol/fluticasone propionate: a review of its use in asthma. Drugs 2005; 65(12): 1715–34
Macdessi JS, Randell TL, Donaghue KC, et al. Adrenal crises in children treated with high-dose inhaled corticosteroids for asthma. Med J Aust 2003; 178(5): 214–6
Todd GR, Acerini CL, Ross-Russell R, et al. Survey of adrenal crisis associated with inhaled corticosteroids in the United Kingdom. Arch Dis Child 2002; 87(6): 457–61
Nelson HS, Weiss ST, Bleecker ER, et al. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest 2006; 129(1): 15–26
Allen & Hanburys. Seretide Evohaler, summary of product characteristics. 2005
Bateman ED, Silins V, Bogolubov M. Clinical equivalence of salmeterol/fluticasone propionate in combination (50/100 microg twice daily) when administered via a chlorofluorocarbonfree metered dose inhaler or dry powder inhaler to patients with mild-to-moderate asthma. Respir Med 2001; 95(2): 136–46
van Noord JA, Lill T, Carillo Diaz T, et al. Clinical equivalence of a salmeterol/fluticasone proprionate combination product (50/500ug) delivered via a chlorofluorocarbon-free metered-dose inhaler with the Diskus™ in patients with moderate to severe asthma. Clin Drug Invest 2001; 21(4): 243–56
Shakir SAW. Causality and correlation in pharmacovigilance. In: Talbot J, Waller P, editors. Stephens’ detection of new adverse drug reactions. 5th ed. Chichester: John Wiley & Sons Ltd, 2003: 329–43
CIOMS/WHO. International ethical guidelines for biomedical research involving human subjects. Geneva: CIOMS/WHO, 2002
Royal College of Physicians of London. Guidelines on the practice of ethical research involving human subjects. London: Royal College of Physicians of London, 1996
General Medical Council. Confidentiality: protecting and providing information. Frequently asked questions Q7. London: General Medical Council, 2004: 9
Heeley E, Riley J, Layton D, et al. Prescription-event monitoring and reporting of adverse drug reactions. Lancet 2001; 358: 1872–3
Martin RM, Kapoor KV, Wilton LV, et al. Underreporting of suspected adverse drug reactions to newly marketed (“black triangle”) drugs in general practice: observational study. BMJ 1998; 317(7151): 119–20
Templeton L, Deehan A, Taylor C, et al. Surveying general practitioners: does a low response rate matter? Br J General Pract 1997; 47: 91–4
Fleming DM, Cross KW, Sunderland R, et al. Comparison of the seasonal patterns of asthma identified in general practitioner episodes, hospital admissions, and deaths. Thorax 2000; 55: 662–5
Wong JY, Zacharin MR, Hocking N, et al. Growth and adrenal suppression in asthmatic children on moderate to high doses of fluticasone propionate. J Paediatr Child Health 2002; 38(1): 59–62
Sim D, Griffiths A, Armstrong D, et al. Adrenal suppression from high-dose inhaled fluticasone propionate in children with asthma. Eur Respir J 2003; 21(4): 633–6
Visser MJ, van der Veer E, Ma DS, et al. Side-effects of fluticasone in asthmatic children: no effects after dose reduction. Eur Respir J 2004; 24(3): 420–5
CSM/MCA. Current problems in pharmacovigilance. 2001; 27: 10
Atkins PJ. Dry powder inhalers an overview. Respir Care 2005; 50(10): 1304–12
Nathan RA, Rooklin A, Schoaf L, et al. Efficacy and tolerability of fluticasone propionate/salmeterol administered twice daily via hydrofluoroalkane 134a metered-dose inhaler in adolescent and adult patients with persistent asthma: a randomized, double-blind, placebo-controlled, 12-week study. Clin Ther 2006; 28(1): 73–85 695
Nelson HS, Wolfe JD, Gross G, et al. Efficacy and safety of fluticasone propionate 44 microg/salmeterol 21 microg administered in a hydrofluoroalkane metered-dose inhaler as an initial asthma maintenance treatment. Ann Allergy Asthma Immunol 2003; 91(3): 263–9
Pearlman DS, Peden D, Condemi JJ, et al. Efficacy and safety of fluticasone propionate/salmeterol HFA 134A MDI in patients with mild-to-moderate persistent asthma. J Asthma 2004; 41(8): 797–806
You-Ning L, Humphries M, Du X, et al. Efficacy and safety of salmeterol/fluticasone propionate delivered via a hydrofluoroalkane metered dose inhaler in Chinese patients with moderate asthma poorly controlled with inhaled corticosteroids. Int J Clin Pract 2005; 59(7): 754–9
Wilton LV, Pearce G, Mann RD. The use of newly marketed drugs in children and adolescents prescribed in general practice. Pharmacoepidemiol Drug Saf 1999; 8 Suppl. 1: S37–45
Maconochie N, Doyle P, Prior S. The National Women’s Health Study: assembly and description of a population-based reproductive cohort. BMC Public Health 2004; 4: 35
Bahri P, Tsintis P. Pharmacovigilance-related topics at the level of the International Conference on Harmonisation (ICH). Pharmacoepidemiol Drug Saf 2005; 14(6): 377–87
Tsintis P, La ME. CIOMS and ICH initiatives in pharmacovigilance and risk management: overview and implications. Drug Saf 2004; 27(8): 509–17
Waller PC, Evans SJ. A model for the future conduct of pharmacovigilance. Pharmacoepidemiol Drug Saf 2003; 12(1): 17–29
Acknowledgements
The Drug Safety Research Unit (DSRU) is an independent charity (No. 327206) that works in association with the University of Portsmouth, Portsmouth, UK. It receives unconditional donations from pharmaceutical companies. The companies have no control over the conduct or the publication of the studies conducted by the DSRU. The DSRU has received such funds from GlaxoSmithKline.
Dr Shakir used to be an employee of GlaxoSmithKline. The other authors have no conflicts of interest that are directly relevant to the content of this study.
We would like to thank all the staff at the DSRU who contributed to the study. We thank the general practitioners who have participated in this study, without whose general support prescription-event monitoring would not be possible. We also thank the Prescription Pricing Division of the National Health Service Business Services Authority for their important participation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Perrio, M.J., Wilton, L.V. & Shakir, S.A.W. A Modified Prescription-Event Monitoring Study to Assess the Introduction of Seretide Evohaler™ in England. Drug-Safety 30, 681–695 (2007). https://doi.org/10.2165/00002018-200730080-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00002018-200730080-00005