Abstract
Regulatory concerns on the ability of an ever-increasing number of non-antiarrhythmic drugs to delay ventricular repolarisation, prolong the corrected QT (QTc) interval and induce potentially fatal ventricular tachyarrhythmias have culminated in the adoption of two, internationally harmonised, regulatory guidelines. On 12 May 2005, the International Conference on Harmonisation (ICH) reached an important milestone when it adopted the final texts for clinical (ICH topic E14) and non-clinical (ICH topic S7B) strategies by which drugs should be investigated for their potential to induce these effects during their development.
ICH E14 provides recommendations to sponsors concerning the design, conduct, analysis and interpretation of clinical studies to assess the potential of a drug to delay cardiac repolarisation. Specifically, it calls for a clinical ‘thorough QT/QTc study’ (typically conducted in healthy volunteers), which is intended to determine whether a drug has a threshold pharmacological effect on cardiac repolarisation, as detected by QT/QTc interval prolongation. The E14 recommendations are generally applicable not only to new drugs that have systemic bioavailability but also to approved drugs when a new dose, route of administration or target population that may result in an increased risk is explored. The guideline provides for exceptions when this study may not be required.
Recognising the fractious relationship between ICH E14 and ICH S7B, and the persistence of a number of issues that may require clarity and/or the emergence of other new scientific issues in the future, the ICH Steering Committee has formed an Implementation Working Group that is charged with providing clarity on aspects of the guideline that are ambiguous and responding to issues on which the sponsors are uncertain. This paper provides a commentary on some of the challenges that are likely to be faced by the sponsors of drugs during the next few years of application of these two guidelines. The adoption of these guidelines has left a number of questions unanswered and raised some new ones. When in doubt, the sponsor should seek formal regulatory clarity before making key decisions that may impact further development, assessment and approval of a new chemical entity. Although the goal of developing drugs with much lower torsadogenic potential and without inappropriate restriction in the use (or even rejection) of potentially beneficial drugs is within sight, it is questionable whether the risk of drug-induced proarrhythmia will be eliminated completely.





Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Shah RR. The significance of QT interval in drug development. Br J Clin Pharmacol 2002; 54: 188–202
Priori SG. Exploring the hidden danger of noncardiac drugs. J Cardiovasc Electrophysiol 1998; 9: 1114–6
Shah RR. Drugs, QT interval prolongation and ICH E14: the need to get it right. Drug Saf 2005; 28: 115–25
Committee for Proprietary Medicinal Products. ‘Points to consider: the assessment of the potential for QT interval prolongation by non-cardiovascular medicinal products’ (CPMP/986/96) [online]. European Medicines Evaluation Agency; 1997 Dec 17; London, UK. Available from URL: http://www.emea.eu.int/pdfs/human/swp/09896en.pdf [Accessed 2005 Aug 8]
International Conference on Harmonisation. The non-clinical evaluation of the potential for delayed ventricular repolarization (QT interval prolongation) by human pharmaceuticals [online]. Available from URL: http://www.ich.org/MediaServer.jser?@_ID=2192&@_MODE=GLB [Accessed 2005 Sep 14]
International Conference on Harmonisation. The clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs [online]. Available from URL: http://www.ich.org/MediaServer.jser?@_ID=1476&@_MODE=GLB [Accessed 2005 Sep 14]
Grisanti S, Morganroth J, Shah RR. A practical approach to cardiac safety. Appl Clin Trial. In press
Food and Drug Administration. Safety warnings regarding use of fentanyl transdermal (skin) patches [online]. Public Health Advisory, 2005, July 15. Available from URL: http://www.fda.gov/cder/drug/advisory/fentanyl.htm [Accessed 2005 Aug 8]
Mauro VF, Bingle JF, Ginn SM, et al. Torsade de pointes in a patient receiving intravenous vasopressin. Crit Care Med 1988; 16: 200–1
Charbit B, Funck-Brentano C, Samain E, et al. QT interval prolongation after oxytocin bolus during surgical induced abortion. Clin Pharmacol Ther 2004; 76: 359–64
Marfella R, Nappo F, De Angelis L, et al. The effect of acute hyperglycaemia on QTc duration in healthy man. Diabetologia 2000; 43: 571–5
Liou SC, Chen C, Wong SY, et al. Ventricular tachycardia after oxytocin injection in patients with prolonged Q-T interval syndrome: report of two cases. Acta Anaesthesiol Sin 1998; 36: 49–52
Beasley CM, Mitchell MI, Dmitrienko AA, et al. The combined use of ibutilide as an active control with intensive electrocardiographic sampling and signal averaging as a sensitive method to assess the effects of tadalafil on the human QT interval. J Am Coll Cardiol 2005; 46: 678–87
Food and Drug Administration. FDA Cardiovascular and Renal Advisory Committee (29 May 2003). Levitra briefing document [online]. Available from URL: http://www.fda.gov/ohrms/dockets/ac/03/briefing/3956B1_05_Bayer-Levitra.pdf [Accessed 2005 Aug 8]
Bass AS, Tomaselli G, Bullingham R, et al. Drugs effects on ventricular repolarization: a critical evaluation of the strengths and weaknesses of current methodologies and regulatory practices. J Pharmacol Toxicol Methods 2005; 52: 12–21
McKibbin JK, Pocock WA, Barlow JB, et al. Sotalol, hypokalaemia, syncope, and torsade de pointes. Br Heart J 1984; 2: 157–62
Cavero I, Crumb W. The use of electrocardiograms in clinical trials: a public discussion of the proposed ICH E14 regulatory guidance. Expert Opin Drug Saf 2005; 4: 795–9
Redfern WS, Carlsson L, Davis AS, et al. Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development. Cardiovasc Res 2003; 58: 32–45
Shryock JC, Song Y, Wu L, et al. A mechanistic approach to assess the proarrhythmic risk of QT-prolonging drugs in preclinical pharmacologic studies. J Electrocardiol 2004; 37Suppl.: 34–9
Joshi A, Dimino T, Vohra Y, et al. Preclinical strategies to assess QT liability and torsadogenic potential of new drugs: the role of experimental models. J Electrocardiol 2004; 37Suppl.: 7–14
Ficker E, Kuryshev YA, Dennis AT, et al. Mechanisms of arsenic-induced prolongation of cardiac repolarization. Mol Pharmacol 2004; 66: 33–44
Kuryshev YA, Ficker E, Wang L, et al. Pentamidine-induced long QT syndrome and block of hERG trafficking. J Pharmacol Exp Ther 2005; 312: 316–23
Wible BA, Hawryluk P, Ficker E, et al. HERG-Lite: a novel comprehensive high-throughput screen for drug-induced hERG risk. J Pharmacol Toxicol Methods 2005; 52: 136–45
Kannankeril PJ, Roden DM, Norris KJ, et al. Genetic susceptibility to acquired long QT syndrome: pharmacologic challenge in first-degree relatives. Heart Rhythm 2005; 2: 134–40
Pfizer Inc. Briefing document for Zeldox capsules. Psychopharmacological Advisory Committee [online]. Rockville (MD): Food and Drug Administration, 2000 Jul 19. Available from URL: http://www.fda.gov/ohrms/dockets/ac/00/backgrd/3619b1a.pdf [Accessed 2005 Sep 2]
Rodriguez I, Kilborn MJ, Liu XK, et al. Drug-induced QT prolongation in women during the menstrual cycle. JAMA 2001; 285: 1322–6
Benton RE, Sale M, Flockhart D, et al. Greater quinidine-induced QTc interval prolongation in women. Clin Pharmacol Ther 2000; 67: 413–8
Abi-Gerges N, Philp K, Pollard C, et al. Sex differences in ventricular repolarization: from cardiac electrophysiology to torsades de pointes. Fundam Clin Pharmacol 2004; 18: 139–51
James AF, Cho SC, Hancox JC. Recent advances in understanding sex differences in cardiac repolarization. Prog Biophys Mol Biol 2005 Jun 24, [Epub ahead of print]
Nakagawa M, Ooie T, Ou B, et al. Gender differences in autonomic modulation of ventricular repolarization in humans. J Cardiovasc Electrophysiol 2005; 16: 278–84
Mansi IA, Nash IS. Ethnic differences in electrocardiographic intervals and axes. J Electrocardiol 2001; 34: 303–7
Ackerman MJ, Tester DJ, Jones GS, et al. Ethnic differences in cardiac potassium channel variants: implications for genetic susceptibility to sudden cardiac death and genetic testing for congenital long QT syndrome. Mayo Clin Proc 2003; 78: 1479–87
Ackerman MJ, Splawski I, Makielski JC, et al. Spectrum and prevalence of cardiac sodium channel variants among black, white, Asian, and Hispanic individuals: implications for arrhythmogenic susceptibility and Brugada/long QT syndrome genetic testing. Heart Rhythm 2004; 1: 600–7
Sarapa N, Morganroth J, Couderc JP, et al. Electrocardiographic identification of drug-induced QT prolongation: assessment by different recording and measurement methods. Ann Noninvasive Electrocardiol 2004; 9: 48–57
Savelieva I, Yi G, Guo X, et al. Agreement and reproducibility of automatic versus manual measurement of QT interval and QT dispersion. Am J Cardiol 1998; 81: 471–7
Azie NE, Adam G, Darpo B, et al. Comparing methods of measurement for detecting drug-induced changes in the QT interval: implications for thoroughly conducted ECG studies. Ann Noninvasive Electrocardiol 2004; 9: 166–74
Malik M. The imprecision in heart rate correction may lead to artificial observations of drug induced QT interval changes. PACE 2002; 25: 209–16
Malik M, Farbom P, Batchvarov V, et al. Relation between QT and RR intervals is highly individual among healthy subjects: implications for heart rate correction of the QT interval. Heart 2002; 87: 220–8
Batchvarov VN, Ghuran A, Smetana P, et al. QT-RR relationship in healthy subjects exhibits substantial intersubject variability and high intrasubject stability. Am J Physiol 2002; 282: H2356–63
Fossa AA, Wisialowski T, Magnano A, et al. Dynamic beat-to-beat modeling of the QT-RR interval relationship: analysis of QT prolongation during alterations of autonomic state versus human ether a-go-go-related gene inhibition. J Pharmacol Exp Ther 2005; 312: 1–11
Patterson SD, on behalf of Pharmaceutical Research and Manufacturers of America QT Statistics Expert Team. Investigating drug-induced QT and QTc prolongation in the clinic: a review of statistical design and analysis considerations. Drug Inf J 2005; 39: 243–66
Webster R, Leishman D, Walker D. Towards a drug concentration effect relationship for QT prolongation and torsades de pointes. Curr Opin Drug Discov Devel 2002; 5: 116–26
Cavero I, Crumb W. Native and cloned ion channels from human heart: laboratory models for evaluating the cardiac safety of new drugs. Eur Heart J 2001; 3Suppl. K: K53–63
Wolpert C, Schimpf R, Veltmann C, et al. Clinical characteristics and treatment of short QT syndrome. Expert Rev Cardiovasc Ther 2005; 3: 611–7
Antzelevitch C. Cardiac repolarization: the long and short of it. Europace 2005; 7Suppl. 2: 3–9
Morganroth J. Comparative efficacy and safety of oral mexiletine and quinidine in benign or potentially lethal ventricular arrhythmias. Am J Cardiol 1987; 60: 1276–81
Kang J, Chen X-L, Wang H, et al. Discovery of a small molecule activator of the human ether-ago-go-related gene (hERG) cardiac K+ channel. Mol Pharmacol 2005; 67: 827–36
Zhou J, Augelli-Szafran CE, Bradley JA, et al. Novel potent human ether-a-go-go-related gene (hERG) potassium channel enhancers and their in vitro antiarrhythmic activity. Mol Pharmacol 2005; 68: 876–84
Kinter LB, Siegl PKS, Bass AS. New preclinical guidelines on drug effects on ventricular repolarization: safety pharmacology comes of age. J Pharmacol Toxicol Methods 2005; 52: 153–8
Lawrence CL, Pollard BE, Hammond TG, et al. Nonclinical proarrhythmia models: predicting torsades de pointes. J Pharmacol Toxicol Methods 2005; 52: 46–59
US FDA. Report on ‘Stagnation and innovation: challenge and opportunity on the critical path to new medical products’. Rockville (MD): Food and Drug Administration, 2004 Mar. Available from URL: http://www.fda.gov/oc/initiatives/criticalpath/whitepaper.pdf [Accessed 2005 Jul 23]
Acknowledgements
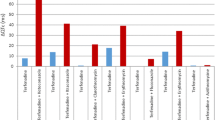
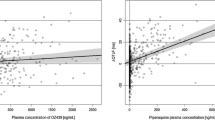
Acknowledgement: I am most grateful to Dr Georg Ferber (Head of Biostatistics Neuroscience, Novartis Pharma AG, Basel, Switzerland and member of the ICH E14 Statistical Group) for allowing me to reproduce from his lectures the three figures that illustrate various computations of drug effect. I also wish to record the pleasure I had in working with all the colleagues of the ICH E14 Expert Working Group as well as the ICH E14 Statistical Group.
Interests: The author was the EU Topic Leader and a member of the ICH E14 Expert Working Group. The author is now a consultant to a number of pharmaceutical companies and also to eResearch Technology, Peterborough, UK. There are no conflicts of interest directly relevant to the contents of this review and the author has not received any funding from any source for this purpose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shah, R.R. Drugs, QTc Interval Prolongation and Final ICH E14 Guideline. Drug-Safety 28, 1009–1028 (2005). https://doi.org/10.2165/00002018-200528110-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00002018-200528110-00003