Abstract
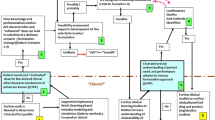
Human safety issues arise throughout the life cycle of pharmaceutical products and relevant information comes from a multitude of sources. Assessment and management of risks to humans requires a problem-based analysis to bring together relevant information regardless of source. The Safety Evaluation Plan (SEP) is a tool to support problem-oriented safety analysis. Safety issues are specified and the evaluation and management of each problem is based on a status summary that integrates the most current information from all relevant sources. The status summary is updated regularly during the course of clinical development to reflect the results of new studies and new clinical trials. In the postmarketing period, relevant postmarketing data is incorporated. Recent regulatory initiatives emphasise early identification of product safety risks so that appropriate risk-management measures can be instituted at the time of approval. A problem-oriented approach supports growing regulatory expectations regarding risk assessment and risk management. The problem-oriented approach facilitates early identification of safety issues and an evidence-based approach to their evaluation. Proactive management of safety problems leads to prompt assessment of risks and timely and appropriate steps aimed at risk reduction. The SEP provides a single global assessment for each safety issue. Regulatory submissions for pharmaceutical and biological products are organised by type of information. International Conference of Harmonisation documents covering clinical safety issues structure and analyse information separately by type, for example, adverse events, serious adverse events, laboratory data, vital signs, etc. A problem-oriented analysis would need to find a place in the regulatory process. A problem-oriented approach to safety cuts across typical structures in the pharmaceutical industry where different groups handle preclinical, clinical and postmarketing safety information. The SEP can improve communication within the company and externally. Nonetheless, supporting structures need to be adapted to support such an interdisciplinary process. Overall, the problem-oriented approach, supported by a SEP, contributes to realistic expectations and sustained credibility when dealing with safety issues.






Similar content being viewed by others
Notes
Although this article focuses on pharmaceutical and biotechnology products, the same considerations apply to medical devices.
References
Kohn L, Corrigan J, Donaldson M, editors. To err is human: building a safer health system. Washington, DC: Committee on Quality of Health Care in America, Institute of Medicine, National Academy Press, 1999
FDA concept paper: premarketing risk assessment [online]. Available from URL: http://www.fda.gov/cder/meeting/riskManageI.htm [Accessed 2003 Mar 3]
FDA concept paper: risk management programs [online]. Available from URL: http://www.fda.gov/cder/meeting/riskManageII.htm [Accessed 2003 Mar 3]
FDA concept paper: risk assessment of observational data: good pharmacovigilance practices and pharmacoepidemiologic assessment [online]. Available from URL: http://www.fda.gov/cder/meeting/riskManageIII.htm [Accessed 2003 Mar 3]
Heads of Agencies Working Group report. Establishing a European risk management strategy: summary report of the Heads of Agencies Ad Hoc Working Group. 2003 Jan
Weed LL. Medical records, medical education, and patient care. Chicago; Year Book Medical Publishers Inc., 1969
Weed LL. Medical records that guide and teach. N Engl J Med 1968; 278(12): 652–7
Roden DM. Drug therapy: drug-induced prolongation of the QT interval. N Engl J Med 2004 Mar; 350(10): 1013–22
Isner JM, Vale PR, Symes JF, et al. Assessment of risks associated with cardiovascular gene therapy in human subjects. Circ Res 2001; 89: 389–400
ICH harmonized tripartite guideline: clinical safety data management. Periodic Safety Update Reports for marketed products E2C. Recommended for adoption at step 4 of the ICH process, 6 Nov, 1996 [online]. Available from URL: http://www.ich.org/MediaServer.jser?.@_ID=477&@_TYPE=MULTIMEDIA&@_TEMPLATE=616&@_MODE=GLB [Accessed 7 May 2004]
McCormack MP, Rabbitts TH. Mechanisms of disease: activation of the T-cell oncogene LMO2 after gene therapy for X-linked severe combined immunodeficiency. N Engl J Med 2004 Feb; 350(9): 913–22
US FDA. BRMAC #34, topic III: briefing document [online]. Available from URL: http://www.fda.gov/ohrms/dockets/ac/03/briefing/3924B2_1.pdf [Accessed 2004 May 3]
Davidson MH. Controversy surrounding the safety of cerivastatin. Expert Opin Drug Saf 2002 Sep; 1(3): 207–12
Silverstein FE, Faich G, Goldstein JL, et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study. A randomized controlled trial: Celecoxib Long-term Arthritis Safety Study. JAMA 2000 Sep 13; 284(10): 1247–55
Knegtering R, Castelein S, Bous H, et al. A randomized open-label study of the impact of quetiapine versus risperidone on sexual functioning. J Clin Psychopharmacol 2004 Feb; 24: 56–61
FDA Center for Drug Evaluation and Research. CDER/CBER risk management public workshop [online]. Available from URL: http://www.fda.gov/cder/meeting/riskManagement.htm [Accessed 2004 May 1]
ICH draft consensus guideline: pharmacovigilance planning (PvP) E2E. Released for consultation at step 2 of the ICH process on 11 November 2003 by the ICH Steering Committee [online]. Available from URL: http://www.ich.org/UrlGrp-Server.jser?.@_ID=276&@_TEMPLATE=254 [Accessed 2004 May 7]
Seligman PJ. Safety issues: regulatory update. Temple University School of Pharmacy/Industry Workshop, 2004 April 20 [online]. Available from URL: http://www.fda.gov/cder/Offices/OPaSS/safetyIssues_files/frame.htm [Accessed 2004 May 7]
Vaillancourt JM, Wallman L. Improve agency/industry communication throughout the drug development process. The 5th Joint Project Management Workshop, Drug Information Association (DIA) and FDA; Bethesda (MD); 2004 May 11-13
ICH Harmonized Tripartate Guidelines. Structure and content of clinical study reports (E3). Recommended adoption at step 4 of the ICH process on 30 Nov, 1995 [online]. Available from URL: http://www.ich.org/MediaServer.jser?.@_ID=479&@_MODE=GLB [Accessed 2004 May 7]
ICH harmonized tripartite guideline: the common technical document for the registration of pharmaceuticals for human use. Quality-M4Q. Quality overall summary of module 2, module 3: quality [online]. Available from URL: http://www.ich.org/MediaServer.jser?.@_ID=556&@_MODE=GLB [Accessed 2004 May 7]
ICH harmonized tripartite guideline: the common technical document for the registration of pharmaceuticals for human use. Safety-M4S. Non-clinical overview and nonclinical summaries of module 2, organization of module 4 [online]. Available from URL: http://www.ich.org/MediaServer.jser?.@_ID=559&@_MODE=GLB [Accessed 2004 May 7]
ICH harmonized tripartite guideline: the common technical document for the registration of pharmaceuticals for human use. Efficacy -M4E. Clinical overview and clinical summary of module 2, module 5: clinical study reports [online]. Available from URL: http://www.ich.org/MediaServer.jser?.@_ID=561&@_MODE=GLB [Accessed 2004 May 7]
Acknowledgements
No sources of funding were used to assist in the preparation of this article. The author has no conflicts of interest that are directly relevant to the content of this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Haas, J.F. A Problem-Oriented Approach to Safety Issues in Drug Development and Beyond. Drug-Safety 27, 555–567 (2004). https://doi.org/10.2165/00002018-200427080-00007
Published:
Issue Date:
DOI: https://doi.org/10.2165/00002018-200427080-00007