Abstract
Pharmaceutical products are not exempt from the practice of counterfeiting. In recent years, many reports have become available demonstrating the presence of fake medicines on the market. Several studies have demonstrated that they are quite often of bad quality.
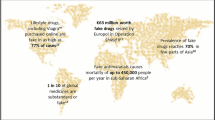
It is estimated that 5% of all world trade in branded goods is counterfeit, leading to huge financial losses for the pharmaceutical industry. But much more important, from a public health point of view, is that history has shown that such products may lead to a great health risk. The essence of counterfeit products and the reason they are so dangerous is the complete absence of quality control, since they are often indistinguishable from the genuine product.
The existence of counterfeit drugs has long been ignored both by the pharmaceutical industry and by drug regulatory authorities. At present initiatives are being taken, nationally and internationally, to curb counterfeiting. It is now realised that a strong regulatory agency is essential, but the initiatives can only be successful if all parties concerned actively co-operate.


Similar content being viewed by others
Notes
In this article, the terms pharmaceutical product, medicinal product, medicine, and drug are equivalent.
A counterfeit medicine is one which is deliberately and fraudulently mislabelled with respect to identity and/or source.[{xc1|1}]
References
Counterfeit Drugs. Report of a joint WHO/IFPMA Workshop. Geneva: World Health Organization, 1992
The Diethylene Glycol Contamination Prevention Workshop. Co-sponsored by CDC, FDA. Washington, DC: PAHO, 1997
Ten Ham M. Counterfeit drugs: implications for health. Adverse Drug React Toxicol Rev 1992; 11(1): 59–65
Banerjee A. Fake polio vaccine kills 11 children in Nadia [newspaper report]. The Asian Age 1995 Apr 5
Cuff R. United Kingdom guidelines on counterfeit drugs. Drug Inf J 1996; 29: 633–7
New anticounterfeiting bill strengthens enforcement. Authentication News 2002 Sep; 8 (7)
Opinion of the Economic and Social Committee on “Counterfeiting” (2001/C211/02). Official Journal of the European Communities, 2001 Aug 7
The Product Surety Center. A resource center for industries and government agencies involved in ensuring the security and safety of products [online]. Available from URL: http://www.productsurety.org [Accessed 2003 Sep 29]
LeParc M, editor. Protecting medicines and pharmaceuticals: a manual of anticounterfeiting solutions. Reconnaissance International, 2002 [online]. Available from URL: http://www.reconnaissance-intl.com/reconnaissance/pharma/index.html [Accessed 2003 Set 29]
Counterfeit Drugs. Report of the International Workshop on Counterfeit Drugs. Geneva, Switzerland: World Health Organization, 1997
Counterfeit Drugs. Guidelines for the development of measures to combat counterfeit drugs. Geneva, Switzerland: World Health Organization, 1999
Bruneton C. Qualité des médicaments génériques antituberculeux au Tchad. Réseau Médicaments & Développement (ReMeD) 1997 Dec: 18
Wondemagnehu E. Counterfeit and substandard drugs in Myanmar and Viet Nam. Report of a study. Geneva: World Health Organization, 1999 (unpublished document)
Poole D. Drug distribution and fake drugs in Nigeria: international workshop. Institute for Medical Informatics. Lagos, Nigeria, 1989
Pakistan tackles counterfeits. SCRIP, 1999
Vietnam: fake drugs sales down sharply. Marketletter 1998 Jan
Acknowledgements
The author declares that no funds were received to assist in the preparation of this article. The author has no conflicts of interest directly relevant to the content of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
ten Ham, M. Health Risks of Counterfeit Pharmaceuticals. Drug-Safety 26, 991–997 (2003). https://doi.org/10.2165/00002018-200326140-00001
Published:
Issue Date:
DOI: https://doi.org/10.2165/00002018-200326140-00001