Abstract
Objective: To evaluate the safety profile of rofecoxib, a selective cyclo-oxygenase-2 inhibitor, in patients with osteoarthritis who are receiving care in non-hospital practice settings.
Design: All patients participating in a large 24-week, open-label, non-pharmacological intervention trial were given rofecoxib for painful osteoarthritis of the knee or hip. They started at a dose of 12.5mg once daily for the first month, with the option of increasing to 25mg daily thereafter, if needed for efficacy. Adverse events were closely monitored. We considered all adverse events that occurred during treatment and within 14 days of discontinuation of rofecoxib.
Patient Group Studied: 2896 patients (861 males and 2035 females) were involved in the safety analysis. Their mean (SD) age was 66.8 (9.9) years, and 631 patients (21.8%) were aged ≥75 years. There were 913 patients (31.5%) with hypertension and 151 (5.2%) with diabetes mellitus at the start of the study; 78 patients (2.7%) had a prior medical history of angina and/or myocardial infarction. The mean (SD) duration of rofecoxib treatment was 139 (62) days.
Results: A total of 519 patients (17.9%) discontinued rofecoxib. The main reasons for discontinuation were dyspepsia (4.4%), nausea (2.4%) and dizziness (2.1%). The annualised incidence rates (95% CI) of complicated and uncomplicated upper gastrointestinal ulcers, myocardial infarction, and stroke were 1.36 (0.76–2.23), 0.09 (0–0.50) and 0.45 (0.16–1.05), respectively.
Conclusion: This study conducted in conditions close to daily practice confirms that the use of rofecoxib is associated with a low rate of serious adverse events in patients with osteoarthritis.




Similar content being viewed by others
References
Bannwarth B. Inhibiteurs sélectifs de COX-2: AINS et estomac enfin réconciliés? Gastroenterol Clin Biol 2001; 25(4 Suppl.): B79–85
Matheson AJ, Figgitt DP. Rofecoxib: a review of its use in the management of osteoarthritis, acute pain and rheumatoid arthritis. Drugs 2001; 61: 833–65
Langman MJ, Jensen DM, Watson DJ, et al. Adverse upper gastrointestinal effects of rofecoxib compared with NSAIDs. JAMA 1999; 282: 1929–33
Bombardier C, Laine L, Reicin A, et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. N Engl J Med 2000; 343: 1520–8
Waller PC. Measuring the frequency of adverse drug reactions. Br J Clin Pharmacol 1992; 33: 249–52
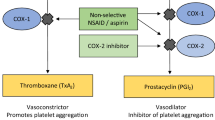
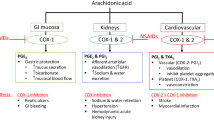
FitzGerald GA, Patrono C. The coxibs, selective inhibitors of cyclooxygenase-2. N Engl J Med 2001; 345: 433–42
Cleland LG, James J, Stamp LK, et al. COX-2 inhibition and thrombotic tendency: a need for surveillance. Med J Aust 2001; 175: 214–7
Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA 2001; 286: 954–9
Howes LG, Krum H. Selective cyclo-oxygenase-2 inhibitors and myocardial infarction: how strong is the link? Drug Saf 2002; 25(12): 829–35
Ravaud P, Giraudeau B, Logeart I, et al. Management of osteoarthritis with patient-administered assessment tools and/or unsupervised home-based exercise program: a 2 × 2 factorial design cluster randomized controlled trial [abstract]. Arthritis Rheum 2001; 44Suppl.: S355
Delamothe T. Reporting adverse drug reactions. BMJ 1992; 304: 465
Naranjo CA, Shear NH, Lanctôt KL. Advances in the diagnosis of adverse drug reactions. J Clin Pharmacol 1992; 32: 897–904
Cannon GW, Caldwell JR, Holt P, et al. Rofecoxib, a specific inhibitor of cyclooxygenase 2, with clinical efficacy comparable with that of diclofenac sodium: results of a one-year, randomized, clinical trial in patients with osteoarthritis of the knee and hip. Arthritis Rheum 2000; 43: 978–87
Crofford LJ, Oates JC, McCune WJ, et al. Thrombosis in patients with connective tissue diseases treated with specific cyclooxygenase 2 inhibitors: a report of four cases. Arthritis Rheum 2000; 43: 1891–6
Reicin AS, Shapiro D, Sperling RS, et al. Comparison of cardiovascular thrombotic events in patients with osteoarthritis treated with rofecoxib versus nonselective nonsteroidal anti-inflammatory drugs (ibuprofen, diclofenac, and nabumetone). Am J Cardiol 2002; 89: 204–9
Sanmuganathan S, Ghahramani P, Jackson PR, et al. Aspirin for primary prevention of coronary heart disease: safety and absolute benefit related to coronary risk derived from meta-analysis from randomised trials. Heart 2001; 85: 265–71
Acknowledgements
This study was funded by Merck Sharp & Dohme-Chibret, Paris, France. The authors thank all French rheumatologists who participated as investigators in this study, and Dr Jean-Sylvain Larguier for his contribution to the analysis of the data.
Professor Bannwarth, Dr Rolland, Professor Ravaud and Professor Dougados have received research support, grants, honoraria and/or consultancy fees from Merck Sharp & Dohme-Chibret, France. No sources of funding were used to assist in the preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bannwarth, B., Trèves, R., Euller-Ziegler, L. et al. Adverse Events Associated with Rofecoxib Therapy. Drug-Safety 26, 49–54 (2003). https://doi.org/10.2165/00002018-200326010-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00002018-200326010-00005