Abstract
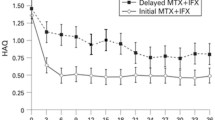
In the last decade, there have been substantial advances in the treatment of rheumatoid arthritis with the addition of several new disease-modifying agents to the therapeutic armamentarium. Biological agents targeting tumour necrosis factor (TNF) represent one such important addition. Infliximab, a chimeric anti-TNF monoclonal antibody, has shown remarkable promise in alleviating the signs and symptoms of rheumatoid arthritis in addition to retarding radiographic disease progression when used in combination with methotrexate. In its pivotal phase III trial, the addition of infliximab to patients with methotrexate-refractory disease was associated with substantial clinical benefit. Using American College of Rheumatology criteria for improvement, one-half of patients receiving infliximab (3 mg/kg every 8 weeks) plus methotrexate showed at least 20% improvement compared with only 20% of those receiving placebo plus methotrexate (p < 0.001) with over one-half of eventual responders obtaining criteria for improvement by the second week of observation. Although its use has been met with much deserved enthusiasm, recent reports have highlighted several potential serious adverse effects associated with infliximab (and other TNF antagonists), including infusion reactions, congestive heart failure, drug-induced lupus, and CNS demyelination. In addition, recent reports have cited the potential for reactivation of mycobacterial and fungal infection in patients receiving infliximab, mandating appropriate tuberculosis screening prior to drug initiation. Although the frequency of serious drug-related toxicity (requiring discontinuation of the agent) appears to be quite low, these reports underscore the need for caution and close surveillance with the administration of TNF inhibitors, particularly given that strategies aimed at preventing toxicity remain unproven. Despite its potential for toxicity, infliximab remains a valuable alternative for patients with rheumatoid arthritis.



Similar content being viewed by others
References
Chu C, Field M, Feldmann M, et al. Localization of tumor necrosis factor alpha in synovial tissues and at the cartilagepannus junction in patients with rheumatoid arthritis. Arthritis Rheum 1991; 34: 1125–32
Saxne T, Palladino M, Heinegard D, et al. Detection of tumor necrosis factor alpha but not tumor necrosis factor beta in rheumatoid arthritis synovial fluid and serum. Arthritis Rheum 1988 Aug; 31 (8): 1041–5
Moreland L, Koopman W. Biological agents as potential therapies for autoimmune diseases. In: Koopman W, editor. Arthritis and allied conditions. 13th ed. Vol. 1. Baltimore: Williams and Wilkins, 1997: 777–809
Keane J, Gershon S, Wise R, et al. Tuberculosis associated with infliximab, a tumor necrosis factor α-neutralizing agent. N Engl J Med 2001; 345: 1098–104
Schwab M, Klotz U. Pharmacokinetic considerations in the treatment of inflammatory bowel disease. Clin Pharmacokinet 2001; 40(10): 723–51
American College of Rheumatology Ad Hoc Committee on Clinical Guidelines. Guidelines for monitoring drug therapy in rheumatoid arthritis. Arthritis Rheum 1996; 39: 723–31
Maini R, Breedveld F, Kalden J, et al. Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum 1998; 41: 1552–63
Schaible T. Treatment of inflammatory diseases: safety of long-term use of infliximab. Presse Med 2001; 30: 610–3
Warner A, Huston K, Scott T, et al. Community experience with infliximab infusion reactions [abstract]. Arthritis Rheum 2001; 44(9): S181
Centocor Inc. Dear healthcare professional [letter]. 2001 Oct 18 [online]. Available from URL: http://www.fda/gov/medwatch/safety/2001/remicade_deardoc.pdf [Accessed 2002 Nov 8]
Maini R, St Clair E, Breedveld F, et al. Infliximab (chimeric anti-tumour necrosis factor α monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. Lancet 1999; 354: 1932–9
Lipsky P, van der Heijde D, St Clair E, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. N Engl J Med 2000; 343: 1594–602
US Food and Drug Administration. Safety update on TNF-α antagonists: infliximab and etanercept. Washington, DC; US Food and Drug Administration, 2002
Charles P, Smeenk R, DeJong J, et al. Assessment of antibodies to double-stranded DNA induced in rheumatoid arthritis patients following treatment with infliximab, a monoclonal antibody to tumor necrosis factor α. Arthritis Rheum 2000; 43: 2383–90
Mohan N, Edwards E, Cupps T, et al. Demyelination occurring during anti-tumor necrosis factor α therapy for inflammatory arthritides. Arthritis Rheum 2001; 44: 2862–9
Robinson W, Genovese M, Moreland L. Demyelinating and neurologic events reported in association with tumor necrosis factor α antagonism. Arthritis Rheum 2001; 44: 1977–83
Elliott M, Maini R, Feldmann M, et al. Treatment of rheumatoid arthritis with chimeric monoclonal antibodies to tumor necrosis factor a. Arthritis Rheum 1993; 36: 1681–90
Fries J, Spitz P, Kraines R, et al. Measurement of patient outcome in arthritis. Arthritis Rheum 1980; 23: 137–45
Elliott M, Maini R, Feldmann M, et al. Repeated therapy with monoclonal antibody to tumour necrosis factor alpha (cA2) in patients with rheumatoid arthritis. Lancet 1994; 344: 1125–7
Elliott M, Maini R, Feldmann M, et al. Randomised double-blind comparison of chimeric monoclonal antibody to tumour necrosis factor a (cA2) versus placebo in rheumatoid arthritis. Lancet 1994; 344: 1105–10
Paulus HE, Egger MJ, Ward JR, et al. Analysis of improvement in individual rheumatoid arthritis patients treated with disease-modifying antirheumatic drugs, based on the findings in patients treated with placebo. Arthritis Rheum 1990; 33: 477–84
Kavanaugh A, St Clair E, McCune W, et al. Chimeric antitumor necrosis factor-alpha monoclonal antibody treatment of patients with rheumatoid arthritis receiving methotrexate therapy. J Rheumatol 2000; 27: 841–50
Felso D, Anderson J, Boers M, et al. The American College of Rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. Arthritis Rheum 1993; 36: 729–40
Puchner T, Kugathasan S, Kelly K, et al. Successful desensitization and therapeutic use of infliximab in adult and pediatric Crohn’s disease patients with prior anaphylactic reactions. Inflamm Bowel Dis 2001; 7: 34–7
Soykan I, Ertan C, Ozden A. Severe anaphylactic reaction to infliximab: report of a case. Am J Gastroenterol 2000; 95: 2395–6
Senaldi G, Yin S. Corynebacterium parvum- and Mycobacterium bovis bacillus Calmette-Guerin-induced granuloma formation is inhibited in TNF I receptor (TNF-RI) knockout mice and by treatment with soluble TNF-RI. J Immunol 1996; 157: 5022–6
Kindler V, Sappino A, Grau G, et al. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell 1989; 56: 731–40
Mohan V, Scanga C, Yu K, et al. Effects of tumor necrosis factor alpha on host immune response in chronic persistent tuberculosis: possible role for limiting pathology. Infect Immunol 2001; 69: 1847–55
Lee JH, Slifman NR, Gershon SK, et al. Life-threatening histoplasmosis complicating immunotherapy with tumour necrosis factor alpha antagonists infliximab and etanercept. Arthritis Rheum 2002; 46: 2565–70
Selmaj K, Raine C, Cross A. Anti-tumor necrosis factor therapy abrogates auto-immune demyelination. Ann Neurol 1991; 30: 694–700
Selmaj K, Papierz W, Glabinski A, et al. Prevention of chronic relapsing experimental auto-immune encephalomyelitis by soluble tumor necrosis factor receptor I. J Neuroimmunol 1995; 56: 135–41
van Oosten B, Barkhof F, Truyen L, et al. Increased MRI activity and immune activation in two multiple sclerosis patients treated with the monoclonal anti-tumor necrosis factor antibody cA2. Neurology 1996; 47: 1531–4
TNF Neutralization in MS. Results of a randomized, placebocontrolled multicenter study. The Lenercept MS Study Group and The University of British Columbia MS/MRI Analysis Group. Neurology 1999; 53: 457–65
Levine B, Kalman J, Mayer L, et al. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med 1990; 26: 236–41
Kubota T, McTiernan C, Frye C, et al. Dilated cardiomyopathy in transgenic mice with cardiac-specific overexpression of tumor necrosis factor-alpha. Circ Res 1997; 81: 627–35
Bozkurt B, Torre-Amione G, Warren M, et al. Results of targeted anti-tumor necrosis factor therapy with etanercept (ENBREL) in patients with advanced heart failure. Circulation 2001; 103: 1044–7
Deswal A, Bozkurt B, Seta Y, et al. Safety and efficacy of a soluble p75 tumor necrosis factor receptor (Enbrel, Etanercept) in patients with advanced heart failure. Circulation 1999; 99: 3224–6
Pugsley M. Etanercept. Immunex. Curr Opin Investig Drugs 2001; 2: 1725–31
Mikuls T, Saag K. Comorbidity in rheumatoid arthritis. Rheum Dis Clin North Am 2001; 27: 283–303
O’Dell J. TNF-α inhibition: the need for a tumor necrosis factor thermostat. Mayo Clin Proc 2001; 76: 573–5
Acknowledgements
Dr Mikuls has no conflicts of interest to report. Dr Moreland is the Director of a large academic Arthritis Clinical Intervention Programme. He has received grant support or served as a consultant for the biotechnology and/or pharmaceutical companies that develop and/or market tumour necrosis factor inhibitors. These include: Centocor, Immunex, Amgen, Wyeth, Pharmacia, Knoll, Abbott, and Johnson and Johnson. Dr Moreland also received grant support and has been a consultant for other companies that are developing antirheumatic medications.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mikuls, T.R., Moreland, L.W. Benefit-Risk Assessment of Infliximab in the Treatment of Rheumatoid Arthritis. Drug-Safety 26, 23–32 (2003). https://doi.org/10.2165/00002018-200326010-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00002018-200326010-00003