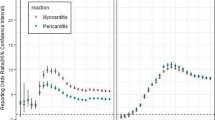
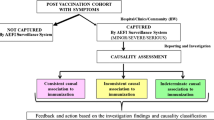
Abstract
The safety of vaccines, particularly the most widely used vaccines to which most children are exposed as infants and toddlers, has always been an extremely high priority for vaccine manufacturers and government agencies. Products intended for healthy people must be held to a high standard of safety assurance. In addition to the intense safety assessments conducted prior to licensure, post-marketing surveillance programmes are essential to identify and study possible risks that occur too rarely to have been identified in pre-licensure studies or that occur in populations not studied in pre-licensure studies. Studying rare risks of vaccines is more complex than for therapeutic products because the exposure is virtually universal for many vaccines, ensuring occurrence simply by chance of many adverse outcomes in temporal association with vaccination. In the US the Vaccine Safety Datalink (VSD), a consortium of managed care organisations, has been established to study more rigourously possible vaccine-associated risks. These risks may be identified through reports to the Vaccine Adverse Event Reporting System (VAERS), the nationwide passive surveillance programme, as well as other sources. The combination of passive surveillance and more structured case-control or cohort studies possible in the VSD has helped to both identify new vaccine risks and to provide reassuring evidence of lack of risk in other situations where concerns have been raised.
Similar content being viewed by others
References
Howson CP, Howe CJ, Fineberg HV, editors. Adverse effects of pertussis and rubella vaccines. Washington, DC: National Academy Press, 1991
Stratton KR, Howe CJ, Johnston RB, editors. Adverse events associated with childhood vaccines: evidence bearing on causality. Washington, DC: National Academy Press, 1994
Stratton KR, Howe CJ, Johnston RB, editors. DPT vaccine and chronic nervous system dysfunction: a new analysis. Washington, DC: National Academy Press, 1994
Stratton K, Gable A, Shetty P, et al., editors. Immunization safety review: measles-mumps-rubella vaccine and autism. Washington, DC: National Academy Press, 2001
Adverse events: surveillance systems for adverse events and medical errors. Testimony before the Subcommittees on Health and Environment, and Oversight and Investigations, Committee on Commerce and Subcommittee on Health, Committee on Veterans’ Affairs, House of Representatives. GA0/T-HEHS-00-61, (2000)
Gangarosa EJ, Galazka AM, Wolfe CR, et al. Impact of antivaccine movements on pertussis control: the untold story. Lancet 1998 Jan 31; 335(9099): 356–61
Medical Dictionary for Regulatory Activities (MedDRA) [online]. Available from URL: http://www.meddramsso.com [Accessed 2002 Mar 4]
COSTART: Coding symbols for thesaurus of adverse reaction terms. 5th ed. Food and Drug Administration, Rockville MD 1995
Baum C, Kweder S, Anello C. The spontaneous reporting system in the United States. In: Strom BL, editor. Pharmacoepidemiology. 2nd ed. Chichester: John Wiley and Sons, 1994: 125–37
Ellenberg SS, Chen RT. The complicated task of monitoring vaccine safety. Public Health Rep 1997 Jan/Feb; 112: 101–9
Parkman PD, Hardegree MC. Regulation and testing of vaccines. In: Plotkin SA, Orenstein WA, editors. Vaccines. 3rd ed. Philadelphia (PA): Saunders, 1999: 1131–43
Ellenberg SS. Safety considerations for new vaccine development. Pharmacoepidemiol Drug Saf 2001; 10: 411–5
Chen RT, Rastogi SC, Mullen JR, et al. The Vaccine Adverse Event Reporting System (VAERS). Vaccine 1994; 12: 542–50
Intussusception among recipients of rotavirus vaccine. United States, 1998–1999. MMWR Morb Mortal Wkly Rep 1999; 48: 577–81
Zanardi LR, Haber P, Mootrey GT, et al. Intussusception among recipients of rotavirus vaccine: reports to the Vaccine Adverse Event Reporting System. Pediatrics 2001; 107(6): E97
Vaccine Adverse Event Reporting System (VAERS) [online]. Available from URL: http://www.vaers.org [Accessed 2002 Mar 4]
Rosenthal S, Chen RT. Reporting sensitivities of two passive surveillance systems for vaccine adverse events. Am J Public Health 1995; 85: 1706–9
DeStefano F. The Vaccine Safety Datalink project. Pharmacoepidemiol Drug Saf 2001; 10: 403–6
Maclure M. The case-crossover design: a method for studying transient reactions associated with intermittent exposures. Am J Epidemiol 1991; 133: 144–53
Davis RL, Kramarz P, Bohlke K, et al. Measles-mumps-rubella and other measles-containing vaccines do not increase risk for bowel disease: a case-control study from the Vaccine Safety Datalink project. Arch Pediatr Adolesc Med 2001 Mar; 155(3): 354–9
Ray P, Black S, Shinefield H. Risk of chronic arthropathy among women after rubella vaccinations. JAMA 1997; 278: 551–6
Kramarz P, Destefano F, Gargiullo PM, et al. Does influenza vaccination prevent asthma exacerbations in children? J Pediatr 2001 Mar; 138(3): 306–10
Ascherio A, Zhang SM, Hernan MA, et al. Hepatitis B vaccination and the risk of multiple sclerosis. N Engl J Med 2001; 344: 327–32
Confavreux C, Suissa S, Saddier P, et al. Vaccinations and the risk of relapse of multiple sclerosis. N Engl J Med 2001; 344: 319–26
Causality assessment of adverse events following immunization. Wkly Epidemiol Rec 2001 Mar; 76 (12): 8509
Dourado I, Cunha S, Teixeira MG, et al. Outbreak of aseptic meningitis associated with mass vaccination with a Urabecontaining measles-mumps-rubella vaccine: implications for immunization programs. Am J Epidemiol 2000 Mar 1; 151(5): 524–30
Murphy TV, Margiullo PM, Mehran S, et al. Intussusception among infants given an oral rotavirus vaccine. N Engl J Med 2001 Feb; 344(8): 564–72
Rennels MB. The rotavirus vaccine story: a clinical investigator’s view. Pediatrics 2000; 106: 123–5
Kramarz P, France EK, Destefano F, et al. Population-based study of rotavirus vaccination and intussusception. Pediatr Infect Dis J 2001 Apr; 20(4): 410–6
Wise RP, Kitonga PK, Salive ME. Hair loss after routine immunizations. JAMA 1997 Oct; 278(14): 1176–8
Meyboom RHB, Hekster YA, Egberts ACG, et al. Causal or casual? The role of causality assessment in pharmacovigilance. Drug Saf 1997; 17: 374–89
DuMouchel W. Bayesian data mining in large frequency tables, with an application to the FDA Spontaneous Reporting System. Am Stat 1999; 53: 177–90
O’Neill RT, Szarfman A. Discussion: Bayesian data mining in large frequency tables, with an application to FDA Spontaneous Reporting System by William DuMouchel. Am Stat 1999; 53: 190–6
Niu MT, Erwin DE, Braun MM. Data mining in the US Vaccine Adverse Event Reporting System (VAERS): early detection of intussusception and other events after rotavirus vaccination. Vaccine 2001; 19: 4627–34
Niu MT, Davis DM, Ellenberg SS. Recombinant hepatitis B vaccination of neonates and infants: emerging safety data from the Vaccine Adverse Event Reporting System. Pediatr Infect Dis J 1996 Oct; 15: 771–6
Niu MT, Rhodes P, Salive ME. Comparative safety of two recombinant hepatitis B vaccines in children: data from the Vaccine Adverse Event Reporting System (VAERS) and Vaccine Safety Datalink (VSD). J Clin Epidemiol 1998; 51(6): 503–10
Acknowledgements
The authors received no external funding for the preparation of this manuscript, and have no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ellenberg, S.S., Braun, M.M. Monitoring the Safety of Vaccines. Drug-Safety 25, 145–152 (2002). https://doi.org/10.2165/00002018-200225030-00001
Published:
Issue Date:
DOI: https://doi.org/10.2165/00002018-200225030-00001