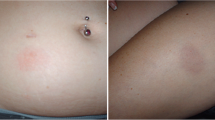
Abstract
Interferon-β is an established therapy in relapsing-remitting multiple sclerosis. Recently, it has also been shown that interferon-β-1b is effective in secondary progressive multiple sclerosis. However, adverse effects of interferon-β treatment are common, particularly during the first weeks of treatment, and are a major concern. Flu-like symptoms, injection site reactions and laboratory abnormalities are the most common adverse effects, and may result in reduced compliance or even discontinuation of treatment in a number of patients. Therefore, efforts to minimise these reactions, e.g. appropriate comedication with analgesic/antipyretic drugs, use of correct preparation and injection technique and sometimes modification of the dosage of interferon-p, are of considerable importance.
This article provides an overview of the management of clinically relevant adverse effects related to treatment with interferon-β, based on a literature review and personal experience. Essential aspects of patient information are also stressed. If these recommendations are followed, adverse effects related to interferon-β may be substantially reduced in the majority of patients.




Similar content being viewed by others
References
The IFNB Multiple Sclerosis Study Group. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology 1993; 43: 655–61
Jacobs LD, Cookfair DL, Rudick RA, et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. Ann Neurol 1996; 39: 285–94
PRISMS (Prevention of Relapses and Disability by Interferon β-1a Subcutaneously in Multiple Sclerosis) Study Group. Randomised double-blind placebo-controlled study of interferon β-1a in relapsing/remitting multiple sclerosis. Lancet 1998; 352: 1498–504
European Study Group on Interferon β-1b in Secondary Progressive MS. Placebo-controlled multicentre randomised trial of interferon β-1b in treatment of secondary progressive multiple sclerosis. Lancet 1998; 352: 1491–7
Betaseron® (interferon beta-1b) product monograph. Richmond (CA): Berlex Laboratories, 1993 [revision date 10/96]
Rebif® (interferon beta-1a) product monograph. Geneva: Ares-Serono International SA, 1998
Avonex™ (interferon beta-1a): brief summary of full prescribing information. Cambridge (MA): Biogen Inc., 1996
Logan-Clubb L, Stacy M. An open-labelled assessment of adverse effects associated with interferon 1-beta in the treatment of multiple sclerosis. J Neurosci Nurs 1995; 27: 344–7
Neilly LK, Goodin DS, Goodkin DE, et al. Side effect profile of interferon beta-1b in MS: results of an open label trial. Neurology 1996; 46: 552–4
Huber S, Spycher M, Lechner-Scott J, et al. Multiple sclerosis: therapy with recombinant beta-1b interferon: initial results with 30 multiple sclerosis patients in northwest Switzerland. Schweiz Med Wochenschr 1996; 126: 1475–81
Schwid SR, Goodman AD, Mattson DH. Autoimmune hyperthyroidism in patients with multiple sclerosis treated with interferon beta-1b. Arch Neurol 1997; 57: 1169–70
Durelli L, Bongioanni MR, Ferrero B, et al. Interferon treatment for multiple sclerosis: autoimmune complications may be lethal. Neurology 1998; 50: 570–1
Rotondi M, Oliviero A, Profice P, et al. Occurence of thyroid autoimmunity and dysfunction throughout a nine-month follow-up in patients undergoing interferon-beta therapy for multiple sclerosis. J Endocrinol Invest 1998; 21: 748–52
Durelli L, Ferrero B, Oggero A, et al. Autoimmune events during interferon beta-1b treatment for multiple sclerosis. J Neurol Sci 1999; 162: 74–83
Blake G, Murphy S. Onset of myasthenia gravis in a patient with multiple sclerosis during interferon-1b treatment. Neurology 1997; 49: 1747–8
Webster GF, Knobler RL, Lublin FD, et al. Cutaneous ulcerations and pustular psoriasis flare caused by recombinant interferon beta injections in patients with multiple sclerosis. J Am Acad Dermatol 1996; 34: 365–70
Mehta CL, Tyler RJ, Cripps DJ. Granulomatous dermatitis with focal sarcoidal features associated with recombinant interferon β-1b injections. J Am Acad Dermatol 1998; 39: 1024–8
Linden D. Severe Raynaud’s phenomenon associated with interferon-β treatmentformultiplesclerosis. Lancet 1998; 352: 878–9
Cohen BA, Greenberger PA, Saini S. Delayed occurence of a severe cutaneous reaction in a multiple sclerosis patient taking interferon beta-1b. Allergy Asthma Proc 1998; 19: 85–8
Nousari HC, Kimyai-Asadi A, Tausk FA. Subacute cutaneous lupus erythematosus associated with interferon beta-1a. Lancet 1998; 352: 1825–6
Jabaily JA, Thompson JS. Effects of interferon beta-1B in rheumatoid arthritis: acasereport. Arthritis Rheum 1997; 40: 1370
Alsalameh S, Manger B, Kern P, et al. New onset of rheumatoid arthritis during interferon β-1B treatment in a patient with multiple sclerosis: comment on the case report by Jabaily and Thompson. Arthritis Rheum 1998; 41: 754
Levesque MC, Ward FE, Jeffery DR, et al. Interferon-β1a-induced polyarthritis in a patient with the HLA-DRB1*0404 allele. Arthritis Rheum 1999; 42: 569–73
Colosimo C, Pozzilli C, Frontoni M, et al. No increase of serum autoantibodies during therapy with recombinant human interferon-β1a in relapsing-remitting multiple sclerosis. Acta Neurol Scand 1997; 96: 372–4
Kivisakk P, Lundahl J, von Heigl Z, et al. No evidence for increased frequency of autoantibodies during interferon-β1b treatment of multiple sclerosis. Acta Neurol Scand 1998; 97: 320–3
Dayal AS, Jensen MA, Lledo A et al. Interferon-gamma-secreting cells in multiple sclerosis patients treated with interferon beta-1b. Neurology 1995; 45: 2173–7
Byskosh PV, Reder AT. Interferon β-1b effects on cytokine mRNA in peripheral mononuclear cells in multiple sclerosis. Mult Scler 1996; 1: 262–9
Panitch HS, Hirsch RL, Haley AS, et al. Exacerbations of multiple sclerosis in patients treated with gamma interferon. Lancet 1987; I: 893–5
The IFNB Multiple Sclerosis Study Group, University of British Columbia MS/MRI Analysis Group. Interferon beta-1b in the treatment of multiple sclerosis: final outcome of the randomized controlled trial. Neurology 1995; 45: 1277–85
Mohr DC, Goodkin DE, Likosky W, et al. Therapeutic expectations of patients with multiple sclerosis upon initiating interferon beta-1b: relationship to adherence to treatment. Mult Scler 1996; 2: 222–6
Walther EU, Dietrich E, Hohlfeld R. Therapy for multiple sclerosis with interferon-beta-1b. Advice to patients, including how to deal with side effects. Nervenarzt 1996; 67: 452–6
Munschauer FE, Kinkel RP. Managing side effects of interferon-beta in patients with relapsing-remitting multiple sclerosis. Clin Ther 1997; 19: 883–93
Lublin FD, Whitaker JN, Eidelman BH, et al. Management of patients receiving interferon beta-1b for multiple sclerosis: report of a consensus conference. Neurology 1996; 46: 12–8
Report of the quality standards subcommittee of the American Academy of Neurology. Practise advisory on selection of patients with multiple sclerosis for treatment with Betaseron. Neurology 1994, 44: 1537–40
Rio J, Nos C, Marzo ME, et al. Low-dose steroids reduce flulike symptoms at the initiation of IFNβ-1b in relapsing-remitting MS. Neurology 1998; 50: 1910–2
Martínez-Cáceres EM, Rio J, Barrau M, et al. Amelioration of flulike symptoms at the onset of interferon-β-1b therapy in multiple sclerosis by low-dose oral steroids is related to a decrease in interleukin-6 induction. Ann Neurol 1998; 44: 682–5
Rieckmann P, Weber F, Gunther A, et al. Pentoxifylline, a phosphodiesterase inhibitor, induces immune deviation in patients with multiple sclerosis. J Neuroimmunol 1996; 64: 193–200
Rieckmann P, Bitsch A, Multiple Sclerosis Study Group. Effect of pentoxifylline on disease activity and immunological markers in multiple sclerosis: results from a double-blind, placebo-controlled, cross-over trial in patients with relapsing remitting MS. Mult Scler 1998; 4: 325
Weber F, Polak T, Günther A, et al. Synergistic immunomodulatory effects of interferon-β1b and the phosphodiesterase inhibitor pentoxifylline in patients with relapsing-remitting multiple sclerosis. Ann Neurol 1998; 44: 27–34
Rieckmann P, Weber F, Gunther A, et al. The phosphodiesterase inhibitor pentoxifylline reduces early side effects of interferon-β1b treatment in patients with multiple sclerosis. Neurology 1996, 47: 604
Skias DD, Reder AT. IL-10 inhibits EAE [abstract]. Neurology 1995; 45: A349
Gaines AR, Varricchio F. Interferon beta-1b injection site reactions and necroses. Mult Scler 1998; 4: 70–3
Rieger-Ziegler V, Kränke B, Soyer P, et al. Large cutaneous-subcutaneous infiltrates: a side effect of interferon beta injections. Hautarzt 1998; 49: 310–2
Elgart GW, Sheremata W, Ahn YS. Cutaneous reactions to recombinant human interferon beta-1b: the clinical and histologic spectrum. J Am Acad Dermatol 1997; 37: 553–8
Weinberg JM, Wolfe JT, Sood S, et al. Cutaneous necrosis associated with recombinant interferon injection. Acta Derm Venereol (Stockh) 1997; 77: 146–8
Albani C, Albani G. A case of cutaneous necrosis during interferon-β1b (B-IFN) therapy in multiple sclerosis. J Neurol Neurosurg Psychiatry 1997; 62: 418
Feldmann R, Löw-Weiser H, Duschet P, et al. Necrotizing cutaneous lesions caused by interferon beta injections in a patient with multiple sclerosis. Dermatology 1997; 195: 52–3
Knobler RL, Kelley CL, Trantas F, et al. Interferon beta-1b induced ulcerative skin lesions in MS. J Neuroimmunol 1994; 54: 173
Bocci V, Pacini A, Bandinelli L, et al. The role of liver in the catabolism of human α- and β-interferon. J Gen Virol 1982; 60: 397–400
Renton KW, Armstrong SG. Immune-mediated downregulation of cytochrome P450 and related drug biotransformation. In: Dean JH, Luster MI, Munson AE, et al., editors. Immunotoxicology and immunopharmacology. New York: Raven Press, 1994: 501–12
Mohr DC, Goodkin DE, Likosky W, et al. Treatment of depression improves adherence to interferon beta-1b therapy for multiple sclerosis. Arch Neurol 1997; 54: 531–3
Joffe RT, Lippert GP, Gray TA, et al. Mood disorder and multiple sclerosis. Arch Neurol 1987; 44: 376–8
Sadovnick AD, Remick RA, Allen J, et al. Depression and multiple sclerosis. Neurology 1996; 46: 628–32
Sadovnick AD, Eisen K, Ebers GC, et al. Cause of deaths in patients attending multiple sclerosis clinics. Neurology 1991; 41: 1193–6
Stenager EN, Stenager E, Koch-Henriksen N, et al. Suicide and multiple sclerosis: an epidemiological investigation. J Neurol Neurosurg Psychiatry 1992; 55: 542–5
Pakulski LA, DiMarco LM. Severe vaginal bleeding associated with recombinant interferon beta-1B. Ann Pharmacother 1997; 31: 50–2
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bayas, A., Rieckmann, P. Managing the Adverse Effects of Interferon-β Therapy in Multiple Sclerosis. Drug-Safety 22, 149–159 (2000). https://doi.org/10.2165/00002018-200022020-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00002018-200022020-00006