Abstract
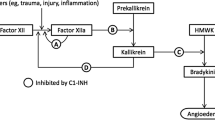
Intravenous nanofiltered human C1 inhibitor (C1-INH NF) concentrate (Cinryze®) is used as a direct replacement of deficient levels of plasma C1 inhibitor in patients with hereditary angioedema (HAE). In the EU, C1-INH NF concentrate 1000 U is indicated in the treatment, pre-procedural prevention, and routine prevention of angioedema attacks in adults and adolescents with HAE.
Intravenous C1-INH NF concentrate 1000 U effectively relieved angioedema attacks in patients with HAE. In a randomized, double-blind trial in pediatric and adult patients, the median time to onset of unequivocal relief from an attack was significantly shorter with C1-INH NF concentrate than with placebo. In an open-label trial, both unequivocal relief and clinical relief were shown in the majority of attacks within 1 and 4 hours of infusion of C1-INH NF concentrate, regardless of the site (i.e. gastrointestinal, cutaneous, laryngeal, or genitourinary) of the defining symptom.
When administered prior to a procedure, open-label intravenous C1-INH NF concentrate 1000 U reduced the incidence of angioedema attacks during and after a variety of dental, surgical, or interventional diagnostic procedures in pediatric and adult patients with HAE.
Routine preventative treatment with intravenous C1-INH NF concentrate 1000 U every 3 or 4 days reduced the number of angioedema attacks. In a randomized, double-blind, crossover trial in pediatric and adult patients with HAE, the mean normalized number of attacks per 12-week period was significantly lower during routine prevention with C1-INH NF concentrate than with placebo. Routine prevention with C1-INH NF concentrate reduced the median monthly attack rate from baseline in an open-label trial.
Intravenous C1-INH NF concentrate was well tolerated in clinical trials in patients with HAE. No cases of viral transmission were reported.






Similar content being viewed by others
References
Bowen T, Cicardi M, Farkas H, et al. 2010 International consensus algorithm for the diagnosis, therapy and management of hereditary angioedema. Allergy Asthma Clin Immunol 2010 Jul 28; 6 (1): 24
Zuraw BL. Hereditary angioedema. N Engl J Med 2008; 359 (10): 1027–36
Bork K. Diagnosis and treatment of hereditary angioedema with normal C1 inhibitor. Allergy Asthma Clin Immunol 2010 Jul 28; 6 (1): 15
Zuraw BL, Busse PJ, White M, et al. Nanofiltered C1 inhibitor concentrate for treatment of hereditary angioedema. N Engl J Med 2010 Aug 5; 363 (6): 513–22
Gösswein T, Kocot A, Emmert G, et al. Mutational spectrum of the C1INH (SERPING1) gene in patients with hereditary angioedema. Cytogenet Genome Res 2008; 121 (3-4): 181–8
Bowen T. Hereditary angioedema: beyond international consensus circa December 2010. The Canadian Society of Allergy and Clinical Immunology Dr. David McCourtie Lecture. Allergy Asthma Clin Immunol 2011 Feb 10; 10 (7): 1
Cinryze 500 units powder and solvent for solution for injection: summary of product characteristics. London: European Medicines Agency, 2011 Jul 15
Guideline on plasma-derived medicinal products. London: European Medicines Agency, 2011 Jul 21
Cinryze: European public assessment report. London: European Medicines Agency, 2011 Jul 15
Cinryze™ (C1 inhibitor, human) for the prophylactic treatment of HAE: briefing document. Blood Products Advisory Committee Meeting. New York (NY): Lev Pharmaceuticals, 2008 May 2
Burnouf T, Padilla A. Current strategies to prevent transmission of prions by human plasma derivatives. Transfus Clin Biol 2006 Nov; 13 (5): 320–8
Burnouf TRM. Nanofiltration of plasma-derived biopharmaceutical products. Haemophilia 2003; 9 (1): 24–37
Kaplan AP. Enzymatic pathways in the pathogenesis of hereditary angioedema: the role of C1 inhibitor therapy. J Allergy Clin Immunol 2010 Nov; 126 (5): 918–25
Davis III AE, Lu F, Mejia P. C1 inhibitor, a multi-functional serine protease inhibitor. Thromb Haemost 2010 Nov 3; 104 (5): 886–93
Riedl M, Zuraw B, Baker J, et al. Open-label use of nanofiltered C1 esterase inhibitor (human) for the treatment of hereditary angioedema attacks [abstract no. 1099]. Allergy 2011 Jun; 66Suppl. 94: 422–3. Plus poster presented at the 30th Congress of the European Academy of Allergy and Clinical Immunology; 2011 Jun 11–15; Istanbul
Zuraw B, Baker J, Hurewitz D, et al. Open-label use of nanofiltered C1 esterase inhibitor (human) (C1 INH-nf) for the prophylaxis of hereditary angioedema (HAE) attacks. [abstract no. P262]. Ann Allergy Asthma Immunol 2010 Nov; 105 (5 Suppl.1): A100. Plus poster presented at the 2010 Annual Meeting of the American College of Allergy, Asthma and Immunology; 2010 Nov 11–16; Phoenix (AZ)
Joseph K, Tholanikunnel TE, Kaplan AP. Treatment of episodes of hereditary angioedema with C1 inhibitor: serial assessment of observed abnormalities of the plasma bradykinin-forming pathway and fibrinolysis. Ann Allergy Asthma Immunol 2010 Jan; 104 (1): 50–4
ViroPharma. A study to evaluate the safety and effect of escalating doses of Cinryze (C1 Inhibitor [Human]) [ClinicalTrials.gov identifier NCT00914966]. US National Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://clinicaltrial.gov [Accessed 2011 Aug 23]
Terpstra FG, Kleinj M, Koenderman AHL, et al. Viral safety of C1-inhibitor NF. Biologicals 2007; 35: 173–81
Lumry W, Baker J, Levy R, et al. Use of nanofiltered C1 esterase inhibitor (human) for the treatment of gastrointestinal attacks in subjects with hereditary angioedema [abstract no. 1088]. Allergy 2011 Jun; 66Suppl. 94: 418–9. Plus poster presented at the 30th Congress of the European Academy of Allergy and Clinical Immunology; 2011 Jun 11–15; Istanbul
Riedl M, Lumry W, Baker J, et al. Use of nanofiltered C1 esterase inhibitor (human) for the treatment of extremity and facial attacks in subjects with hereditary angioedema [abstract no. 1095]. Allergy 2011 Jun; 66Suppl. 94: 421. Plus poster presented at the 30th Congress of the European Academy of Allergy and Clinical Immunology; 2011 Jun 11–15; Istanbul
Riedl M, Baker J, Hurewitz D, et al. Safety and efficacy of nanofiltered C1 esterase inhibitor (human) (Cinryze™) for the treatment of laryngeal attacks in subjects with hereditary angioedema (HAE) [abstract no. 902]. J Allergy Clin Immunol 2011 Feb; 127 (2 Suppl.): AB234. Plus poster presented at the 67th Annual Meeting of American Academy of Allergy, Asthma and Immunology; 2011 Mar 18–22; San Francisco (CA)
Lumry W, Baker J, Davis-Lorton M, et al. Open-label use of nanofiltered C1 esterase inhibitor (human) (C1 INH-nf) for treatment of acute attacks of hereditary angioedema (HAE) in pediatric subjects [abstract no. P264]. Ann Allergy Asthma Immunol 2010 Nov; 105 (5 Suppl.1): A100–1. Plus poster presented at the 2010 Annual Meeting of the American College of Allergy, Asthma and Immunology; 2010 Nov 11–16; Phoenix (AZ)
Baker J, Riedl M, Banerji A, et al. Open-label use of nanofiltered C1 esterase inhibitor (human) (C1 INH-nf) for the treatment or prophylaxis of acute attacks of hereditary angioedema (HAE) in pregnant subjects [abstract no. 27]. Ann Allergy Asthma Immunol 2010 Nov; 105 (5 Suppl.1): A10. Plus poster presented at the 2010 Annual Meeting of the American College of Allergy, Asthma and Immunology; 2010 Nov 11–16; Phoenix (AZ)
Lumry WR, Busse P, Baker J, et al. Pre-procedure administration of C1 esterase inhibitor (human) (Cinryze™) for the prevention of hereditary angioedema (HAE) attacks after medical, dental, or surgical procedures [abstract no. 903]. J Allergy Clin Immunol 2011 Feb; 127 (2 Suppl.): AB234. Plus poster presented at the 67th Annual Meeting of American Academy of Allergy, Asthma and Immunology; 2011 Mar 18–22; San Francisco (CA)
Hurewitz D, Grant JA, Busse P, et al. Open-label use of nanofiltered C1 esterase inhibitor (human) (Cinryze™) for the prophylaxis of attacks of hereditary angioedema (HAE) in pediatric subjects [abstract no. P263]. Ann Allergy Asthma Immunol 2010 Feb; 105 (5 Suppl. 1): A100
Bernstein JA, Ritchie B, Levy RJ, et al. Hereditary angioedema: validation of the end point time to onset of relief by correlation with symptom intensity. Allergy Asthma Proc 2011; 32 (1): 36–42
Cinryze (C1 esterase inhibitor [human]) freeze dried powder: US prescribing information. Exton (PA): ViroPharma Inc., 2011 Jan
Longhurst HJ, Farkas H, Craig T, et al. HAE international home therapy consensus document. Allergy Asthma Clin Immunol 2010 Jul 28; 6 (1): 22
Levi M, Choi G, Picavet C, et al. Self-administration of C1-inhibitor concentrate in patients with hereditary or acquired angioedema caused by C1-inhibitor deficiency. J Allergy Clin Immunol 2006; 117 (4): 904–8
Bygum A, Andersen KE, Mikkelsen CS. Self-administration of intravenous C1-inhibitor therapy for hereditary angioedema and associated quality of life benefits. Eur J Dermatol 2009 Mar 30; 19 (2): 147–51
Kreuz W, Rusicke E, Martinez-Saguer I, et al. Home therapy with intravenous human C1-inhibitor in children and adolescents with hereditary angioedema. Transfusion. Epub 2011 Jul 14
Landmesser LM, Tillotson G, Mariano D. Site of care of nanofiltered C1 esterase inhibitor [human] (Cinryze™) in patients with hereditary angioedema (HAE) [abstract no. P315]. Ann Allergy Asthma Immunol 2010 Nov; 105 (5 Suppl. 1): A113
Acknowledgments and Disclosures
This manuscript was reviewed by: L. Bouillet, Internal Medicine Department, National Reference Centre for Angioedema, Grenoble University Hospital, Grenoble, France; H. Farkas, 3rd Department of Internal Medicine, Semmelweis University, Budapest, Hungary; H.J. Longhurst, Department of Immunology, Barts and the London NHS Trust, London, UK.
The preparation of this review was not supported by any external funding. During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on this article. Changes resulting from comments received were made by the author on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lyseng-Williamson, K.A. Nanofiltered Human C1 Inhibitor Concentrate (Cinryze®). BioDrugs 25, 317–327 (2011). https://doi.org/10.2165/11208390-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11208390-000000000-00000