Abstract
Oral fingolimod (Gilenya™), a sphingosine 1-phosphate (S1P) receptor agonist, is the first oral agent and the first in a novel class of disease-modifying therapies (DMTs) to be approved for use in the US for the treatment of relapsing forms of multiple sclerosis (MS). In the EU, fingolimod is approved for use as a single-agent DMT in selected patients with highly-active, relapsing-remitting (RR) MS. This article reviews the pharmacological properties and clinical use of the drug in patients with RRMS.
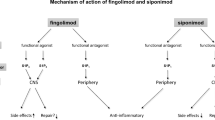
Fingolimod is rapidly converted in vivo to the active moiety S-fingolimodphosphate, which binds with high affinity to S1P receptors, thereby sequestering lymphocytes in the lymph nodes and preventing their egress into the peripheral circulation. As a consequence, there is a reduction in the infiltration of auto-aggressive lymphocytes into the CNS. Fingolimod-phosphate also acts as a functional antagonist, as its binding to S1P receptors results in their internalization and degradation, thereby downregulating S1P receptors on the lymphocyte cell surface. Since fingolimod crosses the blood: brain barrier, it also potentially acts at S1P receptors on neural cells in the CNS to mitigate neuropathological processes associated with MS.
In large multinational trials in adult patients with RRMS, oral fingolimod 0.5mg/day was more effective than oral placebo (FREEDOMS) and recommended dosages of intramuscular interferon-β (IFNβ)-1a (TRANSFORMS) in reducing the annualized relapse rate and was also generally more effective at slowing progression of neurological disability and at reducing the burden and activity of disease. Fingolimod was generally well tolerated in these trials of up to 2 years’ duration, with most adverse events being manageable and of mild to moderate severity; there were two deaths from opportunistic infections, albeit these occurred with fingolimod 1.25mg/day (higher than the recommended dosage). Limited long-term data indicated that no new safety concerns had arisen after 5 years of fingolimod treatment. However, further clinical experience is required to fully determine the long-term safety profile of fingolimod, particularly with regard to any potentially serious or life-threatening adverse events. In the absence of robust pharmacoeconomic studies and of head-to-head trials comparing fingolimod with other formulations of IFNβ and glatiramer acetate, the relative position of fingolimod with respect to other DMTs remains to be fully determined. In the meantime, given its convenient once-daily oral treatment regimen and better efficacy than intramuscular IFNβ-1a, fingolimod is a valuable emerging option for the treatment of adult patients with relapsing forms of MS.







Similar content being viewed by others
References
World Health Organization. Atlas: multiple sclerosis resources in the world 2008 [online]. Available from URL: http://www.who.int/mental_health/neurology/Atlas_MS_WEB.pdf [Accessed 2011 May 2]
Menge T, Weber MS, Hemmer B, et al. Disease-modifying agents for multiple sclerosis: recent advances and future prospects. Drugs 2008; 68(17): 2445–68
Kieseier BC, Wiendl H. Oral disease-modifying treatments for multiple sclerosis: the story so far. CNS Drugs 2007; 21(6): 483–502
World Health Organization. Neurological disorders: a public health approach [online]. Available from URL: http://www.who.int/mental_health/neurology/chapter_3_a_neuro_disorders_public_h_challenges.pdf [Accessed 2011 May 3]
Noseworthy JH, Lucchinetti C, Rodriguez M, et al. Multiple sclerosis. New Engl J Med 2000; 343(13): 938–52
Ryan M, Deno S, Zwibel HL. Review of clinical debate regarding interventions for multiple sclerosis. J Manag Care Pharm 2009; 15(1 Suppl. S-b): S1–17
Lipsy R, Schapiro RT, Prostko CR. Current and future directions in MS management: key considerations for managed care pharmacists. J Manag Care Pharm 2009; 15(9 Suppl. 9-a): S2–15
Lipsy RJ. Will the newer oral MS agents be welcomed by managed care organizations? Am J Manag Care 2010; 16(8 Suppl. ): S227–33
Gold R. Oral therapies for multiple sclerosis: a review of agents in phase III development or recently approved. CNS Drugs 2011; 25(1): 37–52
Brinkmann V. Sphingosine 1-phosphate receptors in health and disease: mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol Ther 2007; 115(1): 84–105
Mehling M, Johnson TA, Antel J, et al. Clinical immunology of the sphingosine 1-phosphate receptor modulator fingolimod (FTY720) in multiple sclerosis. Neurol 2011; 76 Suppl. 3: S20–7
Hla T, Brinkmann V. Sphingosine 1-phosphate (S1P): physiology and the effects of S1P receptor modulation. Neurology 2011; 76(8 Suppl. 3): S3–8
Novartis Pharmaceuticals Corporation. US prescribing information: Gilenya™ (fingolimod) capsules [online]. Available from URL: http://www.pharma.us.novartis.com/product/pi/pdf/gilenya.pdf [Accessed 2010 Dec 8]
European Medicines Agency. Gilenya: summary of product characteristics [online]. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002202/WC500104528.pdf [Accessed 2011 May 15]
Kovarik JM, Schmouder RL, Slade AJ. Overview of FTY720 clinical pharmacokinetics and pharmacology. Ther Drug Monit 2004; 26(6): 585–7
Brinkmann V. FTY720 (fingolimod) in multiple sclerosis: therapeutic effects in the immune and the central nervous system. Br J Pharmacol 2009; 158(5): 1173–82
Chun J, Hartung H-P. Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin Neuropharmacol 2010; 33(2): 91–101
Brinkmann V, Davis MD, Heise CE, et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem 2002; 277(24): 21453–7
Lee CW, Choi JW, Chun J. Neurological S1P signaling as an emerging mechanism of action of oral FTY720 (fingolimod) in multiple sclerosis. Arch Pharm Res 2010; 33(10): 1567–74
Albert R, Hinterding K, Brinkmann V, et al. Novel immunomodulator FTY720 is phosphorylated in rats and humans to form a single stereoisomer: identification, chemical proof, and biological characterization of the biologically active species and its enantiomer. J Med Chem 2005; 48(16): 5373–7
Mandala S, Hajdu R, Bergstrom J, et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science 2002; 296(5566): 346–9
Baumruker T, Billich A, Brinkmann V. FTY720, an immunomodulatory sphingolipid mimetic: translation of a novel mechanism into clinical benefit in multiple sclerosis. Expert Opin Investig Drugs 2007; 16(3): 283–9
Webb M, Tham CS, Lin FF, et al. Sphingosine 1-phosphate receptor agonists attenuate relapsing-remitting experimental autoimmune encephalitis in SJL mice. J Neuroimmunol 2004; 153(1–2): 108–21
Fujino M, Funeshima N, Kitazawa Y, et al. Amelioration of experimental autoimmune encephalomyelitis in Lewis rats by FTY720 treatment. J Pharmacol Exp Therap 2003; 305(1): 70–7
Kataoka H, Sugahara K, Shimano K, et al. FTY720, sphingosine 1-phosphate receptor modulator, ameliorates experimental autoimmune encephalomyelitis by inhibition of T cell infiltration. Cell Mol Immunol 2005; 2(6): 439–48
Foster CA, Mechtcheriakova D, Storch MK, et al. FTY720 rescue therapy in the dark agouti rat model of experimental autoimmune encephalomyelitis: expression of central nervous system genes and reversal of blood-brain-barrier damage. Brain Pathol 2009; 19(2): 254–66
Kovarik JM, Schmouder R, Barilla D, et al. Multiple-dose FTY720: tolerability, pharmacokinetics, and lymphocyte responses in healthy subjects. J Clin Pharmacol 2004; 44: 532–7
Kovarik JM, Schmouder R, Barilla D, et al. Single-dose FTY720 pharmacokinetics, food effect, and pharmacological responses in healthy subjects. Br J Clin Pharmacol 2004; 57(5): 586–91
Mehling M, Lindberg R, Raulf F, et al. Th17 central memory T cells are reduced by FTY720 in patients with multiple sclerosis. Neurology 2010; 75(5): 403–10
Mehling M, Brinkmann V, Antel J, et al. FTY720 therapy exerts differential effects on T cell subsets in multiple sclerosis. Neurology 2008; 71(16): 1261–7
Johnson TA, Lapierre Y, Bar-Or A, et al. Distinct properties of circulating CD8+ T cells in FTY720-treated patients with multiple sclerosis. Arch Neurol 2010; 67(12): 1449–55
Mehling M, Hilbert P, Fritz S, et al. Antigen-specific adaptive immune responses in fingolimod-treated multiple sclerosis patients. Ann Neurol 2011; 69: 408–13
Schmouder R, Boulton C, Wang N, et al. Effects of fingolimod on antibody response following steady-state dosing in healthy volunteers: a 4-week, randomized, placebo-controlled study [abstract plus poster no. P412]. 26th Congress of the European Committee for Treatment and Research in Multiple Sclerosis; 2010 Oct 13–16; Gothenburg
Miron VE, Jung CG, Kim HJ, et al. FTY720 modulates human oligodendrocyte progenitor process extension and survival. Ann Neurol 2008; 63(1): 61–71
Coelho RP, Payne SG, Bittman R, et al. The immunomodulator FTY720 has a direct cytoprotective effect in oligodendrocyte progenitors. J Pharm Exp Ther 2007; 323(2): 626–35
Miron VE, Ludwin SK, Darlington PJ, et al. Fingolimod (FTY720) enhances remyelination following demyelination of organotypic cerebellar slices. Am J Pathol 2010; 176(6): 2682–94
Van Doorn R, Van Horssen J, Verzijl D, et al. Sphingosine 1-phosphate receptor 1 and 3 are upregulated in multiple sclerosis lesions. Glia 2010; 58(12): 1465–76
Kowarik MC, Pellkofer H, Cepok S, et al. Differential effects of fingolimod (FTY720) on immune cells in the CSF and blood of patients with MS. Neurology 2011; 76(14): 1214–21
Kovarik JM, Hartmann S, Bartlett M, et al. Oralintravenous crossover study of fingolimod pharmacokinetics, lymphocyte responses and cardiac effects. Biopharma Drug Dispos 2007; 28(2): 97–104
Burtin P, Ocwieja M, David OJ, et al. Effects of fingolimod on platelet function following steady-state dosing in healthy volunteers: a 4-week, randomized, placebo-controlled study [abstract plus poster no. P445]. 26th Congress of the European Committee for Treatment and Research in Multiple Sclerosis; 2010 Oct 13–16; Gothenburg
Schmouder R, Slade A, Kovarik J, et al. Effects on heart rate when oral fingolimod (FTY720) treatment was combined with atenolol, diltiazem, atropine or isoproterenol [abstract no. P 454]. 25th Congress of the European Committee for Treatment and Research in Multiple Sclerosis; 2009 Sep 9–12; Dusseldorf
Kahan BD, Karlix JL, Ferguson RM, et al. Pharmacodynamics, pharmacokinetics, and safety of multiple doses of FTY720 in stable renal transplant patients: a multicenter, randomized, placebo-controlled, phase I study. Transplantation 2003; 76(7): 1079–84
Zollinger M, Gschwind H-P, Jin Y, et al. Absorption and disposition of the sphingosine 1-phosphate receptor modulator fingolimod (FTY720) in healthy volunteers: a case of xenobiotic biotransformation following endogenous metabolic pathways. Drug Metab Dispos 2011; 39(2): 199–207
Slade AJ, Schmouder RL, Kovarik JM, et al. Oral fingolimod (FTY720) pharmacokinetics and pharmacodynamics are similar between Caucasian and Asian subjects [abstract no. P515]. Multiple Scler 2008; 14 Suppl. : S179–80
Kovarik JM, Schmouder RL, Serra D, et al. FTY720 pharmacokinetics in mild to moderate hepatic impairment. J Clin Pharmacol 2005; 45: 446–52
Kovarik JM, Schmouder RL, Hartmann S, et al. Fingolimod (FTY720) in severe hepatic impairment: pharmacokinetics and relationship to markers of liver function. J Clin Pharmacol 2006; 46(2): 149–56
Kappos L, Radue E-W, O’Connor P, et al. A placebocontrolled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 2010; 362(5): 387–401
Kappos L, Antel J, Comi G, et al. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med 2006; 355(11): 1124–40
Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 2010; 362(5): 402–15
O’Connor P, Comi G, Montalban X, et al. Oral fingolimod (FTY720) in multiple sclerosis: two-year results of a phase II extension study. Neurology 2009; 72(1): 73–9
Comi G, O’Connor P, Montalban X, et al. Phase II study of oral fingolimod (FTY720) in multiple sclerosis: 3-year results. Mult Scler 2010; 16(2): 197–207
Montalban X, O’Connor P, Antel J, et al. Oral fingolimod (FTY720) shows sustained low rates of clinical and MRI disease activity in patients with relapsing multiple sclerosis: four-year results from a phase II extension [abstract no. P06.128]. 61st Annual Meeting of the American Academy of Neurology; 2009 Apr 25–May 2; Seattle (WA)
Izquierdo G, O’Connor P, Montalban X, et al. Long-term fingolimod (FTY720) in relapsing MS: 5 years results from an extension of a phase II, multicenter study show a sustained low level of disease activity [abstract plus poster no. PD6.004]. 63rd Annual Meeting of the American Academy of Neurology (AAN); 2011 Apr 9–16; Honolulu (HI)
Khatri B, Barkhof F, Comi G, et al. Comparison of fingolimod with interferon beta-1a in relapsing-remitting multiple sclerosis: a randomised extension of the TRANSFORMS study. Lancet Neurol 2011; 10(6): 520–9
Cohen J, Barkhof F, Comi G, et al. Oral Fingolimod (FTY720) treatment improves the performance of daily activities compared with intramuscular interferon β-1a: Patient-Reported Indices for Multiple Sclerosis (PRIMUS)-Activities results from a phase III Study (TRANSFORMS) [abstract no. P06.137]. 62nd Annual Meeting of the American Academy of Neurology; 2010 Apr 10–17; Toronto (ON)
McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the Diagnosis of Multiple Sclerosis. Ann Neurol 2001; 50(1): 121–7
Haas J, Hartung HP, Von Rosenstiel P, et al. Effect of fingolimod on severe multiple sclerosis relapses, healthcare utilization and recovery: results from two phase 3 studies, TRANSFORMS and FREEDOMS [abstract plus poster. no P06.049]. 63rd Annual Meeting of the American Academy of Neurology; 2011 Apr 9–16; Honolulu (HI)
Leypoldt F, Münchau A, Moeller F, et al. Hemorrhaging focal encephalitis under fingolimod (FTY720) treatment: a case report. Neurology 2009; 72(11): 1022–4
Schwarz A, Korporal M, Hosch W, et al. Critical vasospasm during fingolimod (FTY720) treatment in a patient with multiple sclerosis. Neurology 2010; 74(24): 2022–4
Uccelli A, Ginocchio F, Mancardi GL, et al. Primary varicella zoster infection associated with fingolimod treatment. Neurology 2011; 76: 1023–4
Zarbin M, Reder AT, Collins W, et al. Ophthalmic evaluations in clinical studies of fingolimod (FTY720) in multiple sclerosis (MS) [abstract plus poster no. PO3.208]. 63rd Annual Meeting of the American Academy of Neurology; 2011 Apr 9–16; Honolulu (HI)
National Institute for Clinical Excellence. Multiple sclerosis: management of multiple sclerosis in primary and secondary care [online]. Available from URL: http://www.nice.org.uk/nicemedia/pdf/cg008guidance.pdf [Accessed 2011 Jun 23]
National Clinical Advisory Board of the National Multiple Sclerosis Society. Disease management consensus statement [online]. Available from URL: http://www.nationalmssociety.org/for-professionals/healthcare-professionals/publications/expert-opinion-papers/index.aspx [Accessed 2011 May 10]
Association of British Neurologists. Revised (2009) Association of British Neurologists’ guidelines for prescribing in multiple sclerosis [online]. Available from URL: http://www.theabn.org/abn/userfiles/file/ABN_MS_Guidelines_2009_Final.pdf [Accessed 2011 May 10]
Goodin DS, Frohman EM, Garmany Jr GP, et al. Disease modifying therapies in multiple sclerosis: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the MS Council for Clinical Practice Guidelines. Neurology 2002; 58(2): 169–78
Goodin DS, Cohen BA, O’Connor P, et al. Assessment: the use of natalizumab (Tysabri) for treatment of multiple sclerosis (an evidence based review). Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2008; 71(10): 766–73
Hohfeld R, Barkhof F, Polman C. Future clinical challenges in multiple sclerosis: relevance of sphingosine 1-phosphate receptor modulator therapy. Neurology 2011; 76(8 Suppl. 3): S28–37
Coyle PK. Early treatment of multiple sclerosis to prevent neurologic damage. Neurology 2008; 71(24 Suppl. 3): S3–7
Novartis Pharmaceuticals. Effect of treatment with fingolimod on the immune response following seasonal flu vaccination and tetanus booster injection in patients with relapsing multiple sclerosis (MS) [ClinicalTrials.gov identifier NCT01199861]. US National Institutes of Health, Clinical-Trials.gov [online]. Available from URL: http://www.clinicaltrials.gov [Accessed 2011 Apr 19]
Merck KGaA. News release. Merck: regulatory update on cladribine tablets [online]. Available from URL: http://news.merck.de/N/0/DAA395BF12E226E6C12578B6007430DB/$File/CladUpdat_e.pdf [Accessed 2011 Jun 23]
European Medicines Agency. Withdrawal of the marketing authorisation application for Movectro (cladribine) [online]. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/Medicine_QA/2011/02/WC500102304.pdf [Accessed 2011 Jun 23]
Gandey A. FDA rejects oral cladribine for multiple sclerosis [online]. Available from URL: http://www.medscape.com/viewarticle/738239_print [Accessed 2011 Jun 23]
Kobelt G, Berg J, Atherly D, et al. Costs and quality of life in multiple sclerosis: a cross-sectional study in the United States. Neurology 2011; 66(11): 1696–702
Author information
Authors and Affiliations
Additional information
Various sections of the manuscript reviewed by: H.-P. Hartung, Department of Neurology, Heinrich-Heine-Universitä, Dusseldorf, Germany; J. Hong, Department of Neurology, Baylor College of Medicine, Houston, TX, USA; B.C. Kieseier, Department of Neurology, Heinrich-Heine-Universitä, Dusseldorf, Germany; T. Menge, Department of Neurology, Heinrich-Heine-Universitä, Dusseldorf, Germany; F. Patti, Department of G.F. Ingrassia, Multiple Sclerosis Centre of Catania University, Catania, Italy; M. Ryan, Pharmacy Practice and Science, University of Kentucky College of Pharmacy, Lexington, KY, USA.
Data Selection
Sources: Medical literature (including published and unpublished data) on ‘fingolimod’ was identified by searching databases since 1996 (including MEDLINE and EMBASE and in-house AdisBase), bibliographies from published literature, clinical trial registries/databases and websites (including those of regional regulatory agencies and the manufacturer). Additional information (including contributory unpublished data) was also requested from the company developing the drug.
Search strategy: MEDLINE, EMBASE and AdisBase search terms were ‘fingolimod’ and (‘multiple sclerosis’ or ‘relapsing remitting multiple sclerosis’ or ‘multiple sclerosis relapsing remitting’). Searches were last updated 6 July 2011.
Selection: Studies in patients with relapsing remitting multiple sclerosis who received fingolimod. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Fingolimod, sphingosine 1-phosphate receptor agonists, multiple sclerosis, relapsing-remitting multiple sclerosis, immunosuppressants, immunomodulators, pharmacodynamics, pharmacokinetics, therapeutic use, tolerability.
Rights and permissions
About this article
Cite this article
Scott, L.J. Fingolimod. CNS Drugs 25, 673–698 (2011). https://doi.org/10.2165/11207350-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11207350-000000000-00000