Summary
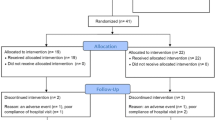
Forty outpatients (20 men and 20 women) aged 60 to 80 years (mean age 69.9 ±5.5 years) with a diagnosis of organic brain psychosyndrome took part in a 3-month randomised, double-blind study aimed at comparing the effectiveness and safety of dihydroergocristine 20mg once daily and placebo. The patients had experienced memory impairment and reduced concentration and clarity of thought, and/or personality and affective disorders for at least 6 months. Patients had a Hachinski Dementia Score ≤15, a Hachinski Ischemic Score <5, and a Hamilton Rating Scale for Depression score of ≤22. The Sandoz Clinical Assessment-Geriatric (SCAG) scale, used as the efficacy variable, was administered on study entry and during (45 days) and after (90 days) treatment. Safety evaluations (routine laboratory tests and measurements of systolic and diastolic blood pressure and heart rate) were performed before and at the end of the study period. All patients fulfilled the entry criteria and completed the study protocol. In the dihydroergocristine group, there was a marked improvement in the SCAG total score and in most partial scores compared with baseline values. Most symptoms had already improved significantly after 45 days of treatment with dihydroergocristine.
In the placebo group, no clinically relevant or statistically significant changes in SCAG scores relative to baseline values were seen at the end of the study period. Safety was assessed as very good in all patients. No adverse events were reported, except for one case of mild self-limiting nausea during dihydroergocristine treatment. No clinically relevant or statistically significant changes relative to baseline values were found in laboratory parameters or in blood pressure and heart rate measurements performed at the end of each treatment. The results of this study suggest that dihydroergocristine 20mg once daily is effective and safe in the treatment of organic brain psychosyndrome.
Similar content being viewed by others
References
Brizzee KR. Neuron aging and neuron pathology. In: Johnson HA, editor. Relations between normal aging and disease. New York: Raven Press, 1985: 191–224
Carlsson A. Aging and neurotrasmitters. In: Platt G, editor. Functionsstorungen des gehirns im alter. Stuttgart: Schattaner, 1981: 67–81
Sohal RS, Allen RG. Relationship between metabolic rate, free radicals, differentiation and aging: a unified theory. In: Woodhead AD, Blackett AD, Hollaender A, editors. The molecular basis of aging. New York: Plenum Press, 1985: 75–104
Sapolsky R, Krey L, McEwen B. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. EndrocrRev 1986; 7: 284–301
Robinson DS. Aging and neuropsychopharmacology. In: Yevit JZ, Bianchine JR, editors. Recent advances in clinical therapeutics. New York: Grune & Stratton Inc., 1983: 127–42
Markstein R. Dopamine receptor profile of co-dergocrine (hydergine) and its components. Eur J Pharmacol 1983; 86: 145–55
Markstein R, Closse A, Frick W. Interaction of ergot alkaloids and their combination with a-adrenoceptors in CNS. Eur J Pharmacol 1983; 93: 159–68
Fiore L, Scapagnini U, Canonico PL. Effect of dihydroergocriptine and dihydroergocristine on cyclic AMP accumulation and prolactin release in vitro: evidence for a dopaminergic action. Hormone Res 1987; 25: 171–7
Moret C, Briley M. Dihydroergocristine-induced stimulation of the 5-HT autoreceptor in the hypothalamus of the rat. Neuropharmacology 1986; 25: 169–74
Gorini A, Arnaboldi R, Vercesi L, et al. Influence of some ergot alkaloids on the cerebral reduced glutathione. Il Farmaco Ed Sci 1988; 43 Suppl. 11: 887–90
Benzi G, Pastoris O, Marzatico F, et al. Influence of aging and drug treatment on the cerebral glutathione system. Neurobiol Aging 1988; 9: 371–5
Coppi G, Zanotti A, Mailland F. Untersuchungen zur Pharmakokinetik von Dihydroergocristin beu freiwillingen Probanden nach oraler Verabreichung von drei Formulierungen. Arzneimittelforsch 1992; 42 Suppl. 11a: 1397–402
Martucci N, Manna V, Mailland F. EEG-pharmacological and neuropsychological study of dihydroergocristine mesylate in patients with chronic cerebrovascular disease. Adv Ther 1986; 3 Suppl. 4: 210–23
Mailland F, Zottino G. Therapeutische effektivitat und Sicherheit von dihydroergocristin. Eine Studie an 9702 patienten. Fortschr Med 1986; 104 Suppl. 11: 239–42
Fioravanti M, Buckley AE, Agnoli A. A multidimensional approach to the assessment of clinical validity in a study on CCVD treatment: dihydroergocristine versus placebo. Arch Gerontol Geriatr 1987; 6: 83–93
Aranda B, Dumoulin P, Groothold G. Kontrollierte Studie zur Wirkung von dihydroergocristin beim hirnorganischen psychosyndrom. Arzneimittelforsch 1992; 42 Suppl. 11a: 1406–9
Lazzaroni M, Cattalini C, Massetto N, et al. Dihydroergocristin beim hirnorganischen psychosyndrome. Multizentrische plazebo-kontrollierte kliniske doppelblindstudie an 240 patienten. Arzneimittelforsch 1992; 42 Suppl. 11a: 1410–3
Mailland F, Poli A, Zottino G. Drug surveillance of dihydroergocristine in long-term use. Curr Ther Res 1987; 42 Suppl. 5: 857–61
Poli M, Caviezel F, Girardi AM, et al. Inhibition of sulpirideinduced prolactin secretion by dihydroergocristine in man. In: Cattabeni F, et al., editors. Long-term effects of neuroleptics. New York: Raven Press, 1980: 445
Poli M, Caviezel F, Bosi E, et al. Cimentine and prolactin: a study with dihydroergocristine. Horm Metab Res 1982; 14: 616–7
Scarduelli C, Cavioni V, Galparoli C, et al. Clinical use of a new antiprolactinemic drug: dihydroergocristine. J Obstet Gynecol 1987; 7: 225–7
Venn RD. The Sandoz Clinical Assessment-Geriatric (SCAG) Scale. A general-purpose phychogeriatric rating scale. Gerontology 1983; 29: 185–98
Yesavage JA, Adey M. Design aspects of clinical trials with ergot alkaloids: a comparison of two geriatric behavioral rating scales. In: Goldstein M, et al., editors. Ergot Compounds and Brain function. New York: Raven Press, 1980: 357
Reisberg B. The brief cognitive rating scale and global deterioration scale. In: Crook T, et al., editors. Assessment in geriatric psychopharmacology. New Canaan: Mark Powley Associates, 1983: 19
Mohs RC, Rosen WG, Greenwald BS, et al. Neuropathological validated scales for Alzheimers disease. In: Crook T, et al., editors. Assessment in geriatric psychopharmacology. New Canaan: Mark Powley Associates, 1983: 37
Salzman C. The Sandoz Clinical Assessment-Geriatric Scale. In: Crook T, et al., editors. Assessment in geriatric psychopharmacology. New Canaan: Mark Powley Associates, 1983: 53
Petrie WM. Psychiatric rating scales for inpatient research. In: Crook T, et al., editors. Assessment in geriatric psychopharmacology. New Canaan: Mark Powley Associates, 1983: 59
Overall JE. Psychiatric rating scales: state of the art and directions for future research. In: Crook T, et al., editors. Assessment in geriatric psychopharmacology. New Canaan: Mark Powley Associates 1983: 49
Rosen WG, Mohs R, Davis KL. Assessing symptom severity in Alzheimers disease. Interdiscip Top Gerontol 1985; 20: 35
Agliati G, Lazzaroni M, Mariani G, et al. Einjährige Therapie mit Dihydroergocristin zur Behandlung von Aufmerksamkeits-und Gedächtnisstörungen bei älteren Patienten. Arzneimittel Forschung 1992; 42 Suppl. 11a: 1414–16
Abate G, Angeleri F, Bartorelli L, et al. Epidemiologische Studie zur Wirksamkeit und Verträglichkeit von Dihydroergocristin bei Verschlechterung der Gedächtnis- und der Verhaltensfunktionen beim älteren Menschen. Arzneimittel Forschung 1992; 42 Suppl. 11a: 1417–21
Malacco E, Di Cesare F. Effect of dihydroergocristine treatment on carbohydrate tolerance and cognitive function in patients with non-insulin-dependent diabetes. Curr Ther Res 1992; 51: 515–23
Franciosi A, Zavattini G. Dihydroergocristine in the treatment of elderly patients with cognitive deterioration: a doubleblind, placebo-controlled, dose-response study. Curr Ther Res 1994; 55: 1391–1401
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Guerzoni, A., Santambrogio, S. Efficacy of Dihydroergocristine 20mg Once Daily in Patients with Organic Brain Psychosyndrome. Clin. Drug Invest. 10, 1–7 (1995). https://doi.org/10.2165/00044011-199510010-00001
Published:
Issue Date:
DOI: https://doi.org/10.2165/00044011-199510010-00001